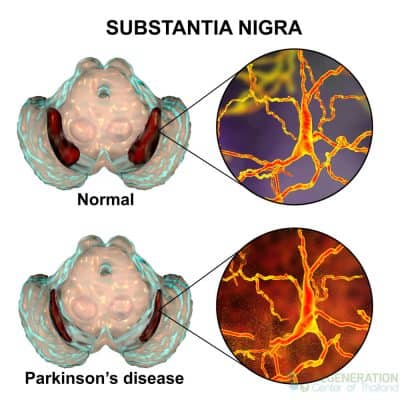
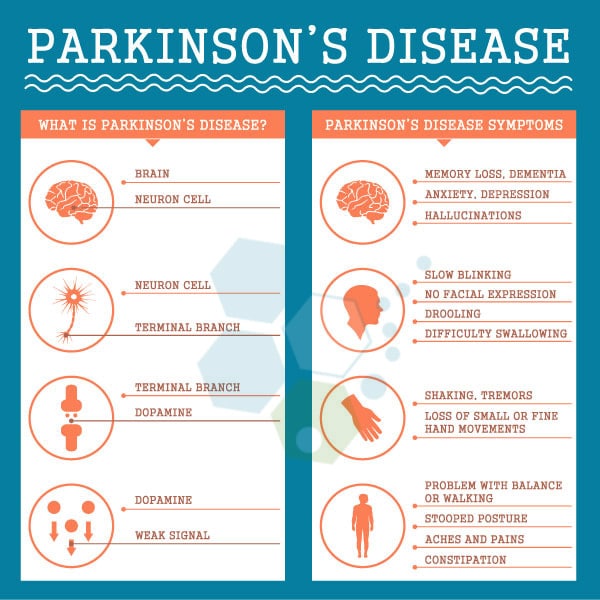
Parkinson’s disease, or “PD,” is a neurodegenerative disorder of the central nervous system. PD is predominantly diagnosed in elderly patients, but 5% of cases happen to patients less than 40 years old. Parkinson’s disease affects more than 6.1 million people worldwide, and patients diagnosed with primary parkinsonism can be characterized by non-motor and motor symptoms.
Breakthrough Therapy for Parkinson’s with Stem Cells
Several types of stem cells are currently being used to treat Sporadic PD. Some of the stem cell-derived therapies have only been studied in clinical trial settings and have been used in Parkinson’s and other neurodegenerative diseases. These stem cell-based therapies include:
- Embryonic Stem Cells (ESCs): These can potentially turn into any cell type in the body. There have been concerns about the ethical implications of using ESCs, and there is the potential for tumor formation when implanted. The Regeneration Center does not offer human embryonic stem cells for Parkinson’s disease.
- Induced Pluripotent Stem Cells (iPSCs): These are cells taken from adults and reprogrammed to act like embryonic stem cells. This technique sidesteps the ethical concerns of using embryonic cells; however, it poses a risk of developing cancers because the IPSC cells can insert DNA anywhere in the cell’s genome, which can trigger the expression of cancer-causing genes. The Regeneration Center does not offer induced pluripotent stem cells (iPSC) stem cell replacement therapy for Parkinson’s
- Isolated Mesenchymal Stem Cells (UC-MSC+): Found in several tissues, including bone marrow, cord tissue, placental tissue, dental pulp (neurogenic), and adipose tissue. Isolated UC-MSC Cells have been successfully used to treat many diseases due to their anti-inflammatory properties. Patients with advanced Parkinson’s disease have limited options when it comes to stem cell sources. The Regeneration Center offers isolated and expanded mesenchymal cells that can home and differentiate into any cell type, including stem cell-derived midbrain dopaminergic neurons.
Common symptoms of Parkinson’s Disease (PD)
The disruptions caused in motor control often include:
- Mild Tremors ( Typically when over 65% of dopamine neurons have degenerated in the substantia nigra.
- Slowness in movement ( bradykinesia )
- Muscle rigidity, movement dysfunctions, and reduction in dopamine-producing cells
- Poor Posture, Motor Function, or Postural instability
History of Parkinsons Disease & Paralysis Agitans
In 1817, a British physician named James Parkinson described in his treatise “An Essay on the Shaking Palsy” the main symptoms of the disease, which was later named after him. Other names and classifications in the Parkinson’s disease family include:
- Shaking Palsy
- Idiopathic Parkinsonism
- Parkinson’s syndrome
- Corticobasal Degeneration (CBD)
- Drug-induced Parkinsonism
- Vascular (Arteriosclerotic) parkinsonism or Multi-infarct parkinsonism
- Progressive supranuclear palsy (PSP)
- Hypokinetic–rigid syndrome (HRS)
- Essential Tremor (ET)
- Paralysis Agitans
What Causes Parkinson’s Disease
For most patients (Over 85% of all cases), the diagnosis of Parkinson’s disease is idiopathic or of unknown origin. Researchers studying Parkinson’s believe that it may be caused by several factors ranging from systemic autoimmune diseases and genetic/familial to environmental causes. Familial Parkinson’s is quite rare, and less than 15% of our patients have reported a family history of Neurodegenerative disorders such as ALS, Ischemic Strokes, Broca’s aphasia, or Ataxia. The primary pathophysiology of Parkinson’s disease is the death of dopaminergic neurons (dopamine-producing cells) and motoneurons in the midbrain (substantia nigra region.) The usual symptoms associated with PD occur due to the loss of paracrine cell signaling capacity of neurotransmitters, especially dopamine. Some other potential risk factors for neurodegenerative diseases like MND and PD include oxidative pressures, autoimmune system complications from disorders such as lupus, hereditary variables, environmental factors, neuroleptics or calcium antagonists, intoxication (Drug-induced parkinsonism), or traumatic brain injuries.
[1]
Is Parkinson’s caused by genetic mutations?
While the risk is low, several genetic mutations can lead to the development of Parkinson’s disease in some individuals. These include mutations in genes such as:
- SNCA – Alpha-synuclein gene
- PRKN – Parkin RBR E3 Ubiquitin Protein Ligase
- PINK1 Gene PARK6
- DJ-1 and PARK7 Gene
- LRRK2
It is believed that mutations in these genes can cause dysfunction and eventual death of dopamine-producing neurons through various mechanisms, including protein aggregation, mitochondrial dysfunction, and oxidative stress. The most common genetic mutation found in Parkinson’s patients is in the LRRK2 gene. However, having one of these mutations does not always mean an individual will develop Parkinson’s – there are likely complex interactions with environmental and other factors at play. Though only a subset of Parkinson’s cases can be attributed to genetic causes, identifying these mutated genes has provided essential insights into the underlying biology of the disease. This could eventually help lead to more targeted and effective treatments. Further research is still needed to understand all the genetic contributors to Parkinson’s disease fully, and the Regeneration Center offers full genetic screening for Parkinson’s and other neurodegenerative disease diseases.
The Role of Lewy Bodies & Symptoms of Parkinson’s Disease
A diagnosis of PD is given when a patient develops a rapid accumulation of alpha-synuclein proteins into the “Lewy bodies” due to the inadequate activity of neurons and dopamine. Dementia with Lewy bodies is reported as the third leading cause after Alzheimer’s and vascular dementia. The four primary symptoms of Parkinson’s disease are bradykinesia, rigors, tremors, and postural instability.
What is Bradykinesia – Bradykinesis?
Bradykinesia means that the patient moves slower and cannot change positions. Activities and motor functions such as getting up, walking, and turning can be challenging. Sometimes, for patients with late-stage Parkinson’s, voluntary movement is almost impossible due to akinesia.
The first sign of PD disease may be that an arm resonates less when walking. Due to frontal gait disorder, the patient’s posture is often bent, and a turn may require many intermediate steps. Gestures and facial expressions decrease; a patient’s face usually looks emotionless, like a mask. The voice becomes quieter and monotonous, and language skills become more indistinct. The person has difficulty eating and is often choking. The handwriting becomes small and illegible.
Limitations of Dopamine Antagonists & Managing Rigor
Rigor describes the stiffness of the muscles. Every movement seems to be against fierce resistance. Often, the neck and shoulder muscles are affected, usually one-sided. A neurologist may be able to detect the Cogwheel phenomenon. An example of cogwheel rigidity can be seen when the doctor attempts to stretch the angled arm of a patient with Parkinson’s, which results in a reaction of jerky movements as if a cogwheel were moving.
Rest tremors
Resting tremors or trembling often occur in patients with Parkinson’s, usually on one side. The slow tremors of the hands and, later, the feet occur primarily during rest periods. The resting tremors often disappear during exercise or sleep. How do you cure brain fog? The tremor is absent in all cases, but many patients report empty hands and fingers rubbing against each other in a movement similar to “counting coins.”
The Role of Physical Exercise & Postural Instability
Postural instability is a primary motor symptom in a later stage of shaking palsy. The lack of balance is especially apparent when the patient is standing upright. PD directly affects the reflexes that help us balance, and patients with postural instability can easily fall with the slightest touch. This symptom is very dangerous for patients and often leads to severe falls, resulting in orthopedic injuries to the hips, knees, shoulders, and arms.
Parkinson’s disease and movement disorders
Parkinson’s disease is a progressive neurological disorder characterized by tremors, stiffness, slow movements, and balance issues. It is caused by the death of dopamine-producing neurons in the substantia nigra region of the Brain. Other joint movement disorders include dystonia (sustained muscle contractions causing abnormal postures), chorea (involuntary jerky movements), tics (sudden, rapid, recurrent motor movements or vocalizations), and restless leg syndrome. Movement disorders can significantly impact the quality of life. A multidisciplinary approach that includes neurologists, physical therapists, occupational therapists, speech therapists, and mental health professionals is essential.
What are some Complications of PD: Akinetic Crisis?
An akinetic crisis (AC) or acute akinesia is a life-threatening complication of PD that can be caused by a variety of issues, including infections, medication errors, or failed surgery. A patient with AC becomes completely immobile, can no longer speak, and can no longer swallow. Accompanying symptoms include sweating and a fast pulse. Such a crisis is dangerous and must be treated immediately. Without the ability to swallow, saliva can enter the lungs and cause pneumonia.
Onset Parkinson’s & Early stages of the disease
When someone aged between 18-50 years old receives a clinical diagnosis of Parkinson’s, it is called young-onset Parkinson’s disease or early-onset Parkinson’s disease. The symptoms of PD are usually the same regardless of age, but younger patients have very different experiences with the condition due to their age. From a psychological, social, and medical standpoint, managing PD is challenging for younger patients and their families.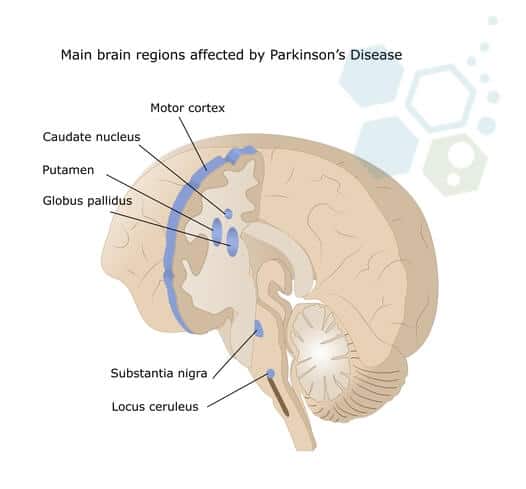 Early symptoms and accompanying symptoms for onset of Parkinson’s disease include:
Early symptoms and accompanying symptoms for onset of Parkinson’s disease include:
- The sense of smell worsens.
- Sleep disorders associated with involuntary and violent movements during REM sleep – (REMS sleep behavior disorder)
- Frequent depression and unhappy moods
- Diffuse muscle pain, reduction in motor function, and pains in the shoulder-arm areas (due to muscle stiffness and limited mobility)
- Excessively oily skin due to hyperactive sebaceous glands in the face
- Issues with body temperature and circulatory system regulation
- Problems with proper bladder and bowel function
- Reduced Cognition
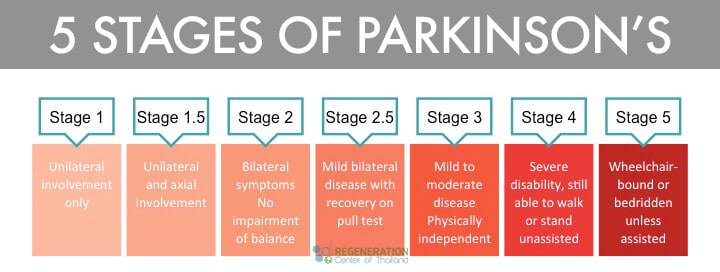
5 Stages of Parkinson’s Disease
Symptoms of Parkinson’s vary from person to person and can take a while to manifest, and the disease is regarded as slow-moving or “insidious onset.” Early symptoms include neuropathy, tremors, and clumsy motions, even when the limbs are at rest. Severe symptoms include muscle rigidity, bradykinesia, and gait disorders. Other non-motor symptoms include incontinent urine, depression, insomnia, and astriction. Neurologists have established the Hoehn and Yahr Scale, which consists of the five stages of PD to help classify patients in clinical trials and research studies worldwide.
Diagnosis of Parkinsonian Syndromes
Diagnosis can be challenging, but patients we have treated at the regeneration center for PD generally don’t show significant abnormalities in lab panels or blood tests. Still, trained neurologists can usually notice irregularities in Cerebrospinal fluid biomarkers through radiography tools such as brain MRI or CT Scan.[2]
The symptoms of Parkinson’s are progressive, but each patient shows a different rate of progression. Losing Motion function causes a decline in the overall quality of life, and drugs have shown minimal effects in clinical trials and treatments worldwide. For severe cases, traditional medicine has virtually no impact and can make it even more challenging to control the symptoms due to new side effects. Severe patients often demonstrate catalepsy and can develop other complications, such as lung respiratory failure, Parkinson’s disease psychosis, and Parkinson’ s-related hallucinations.
Pharmaceutical-based solutions are ineffective for symptomatic patients and cannot alter the course of the degenerative disorder. Drug-based treatments cannot raise the number of neuron cells, thus making most of the traditional treatment options ineffective.
Genetic Testing for Parkinson’s
Hereditary neurodegenerative diseases occur when genetic mutations are passed from the parents to their children. A genetic disease, however, can be due to environmental causes or hereditary. Random mutations can cause genetic conditions in the DNA due to environmental factors or exposure to toxins. Familial (hereditary) PD accounts for less than 15% of all confirmed cases. Inherited parkinsonism is either an X-linked dominant pattern or an autosomal recessive pattern. Each parent carries one copy of the mutated gene but often shows no signs or symptoms. For this reason, autosomal recessive PD can frequently be misdiagnosed as dementia with Lewy bodies, multiple system atrophy (MSA), or ALS/MND. The most common genetic markers used for screening hereditary Parkinsons include LRRK2, Glucocerebrosidase (GBA), RIC3, PARK2, PARK6, PARK7, SYNJ1, PTRHD1, TMEM230, UCHL1, DNAJC6, CYP2D6, VPS13C, PODXL, DNAJC13, CHCHD2, and RAB39B. Recent research also suggests that abnormalities in tau proteins are also a potential cause of familial parkinsonism and frontotemporal dementia.[3]
The Regeneration Center offers gene sequencing tests for 18 of the most common gene causes associated with Parkinson’s disease and monogenic parkinsonism-related conditions. The genes we can test for include: SLC6A3, LRRK2, DNAJC6, FBXO7, GCH1, PARK7, VPS35, PINK1, PRKRA, PRKN, SNCA, SPR, TH, CHCHD2, MAPT, ATP13A2, ATP7B & DCTN1
Please note we do not offer gene therapy for Parkinson’s disease but offer gene testing services for existing patients looking to confirm a diagnosis or who are displaying classical signs of Parkinsonism and can benefit from molecular diagnostic testing. The panels offered by the Regen Center cover genes with recessive, dominant, and X-linked inheritance patterns, which help clarify the risk of recurrence and help to determine which relatives might be at risk based on the family history.
TREATMENT RISKS & PRECAUTIONS
Please note that not all patients are suitable candidates for treating Parkinson’s Disease with stem cells. Patients with advanced motor symptoms, severe cognitive decline, or other significant health conditions might not be good candidates for treatment. The Regeneration Center does not offer embryonic stem cells or induced pluripotent stem cells for the treatment of Parkinson’s diseaseTreatment Options for Parkinson’s in 2025
Parkinson’s disease and some of the symptoms are treatable, but Parkinson’s cure using gene therapy is not available. Recent developments in gene editing and exosome therapy do offer hope for a potential treatment for Parkinson’s. However, the research is still in its infancy and has not proven consistent. Traditional therapies for PD consist of several components to counter low levels of dopamine neurotransmitters in the Brain, with the most common one being pharmaceutical medications. Anti-Parkinson medications are effective for some patients, allowing the disease to be under control for many years. Over time, however, the effect of new Parkinson’s drugs may decrease. Therefore, frequent adjustments of the medications are necessary at specific intervals to ensure the treatments remain effective.
The most common medications for Parkinson’s Disease include: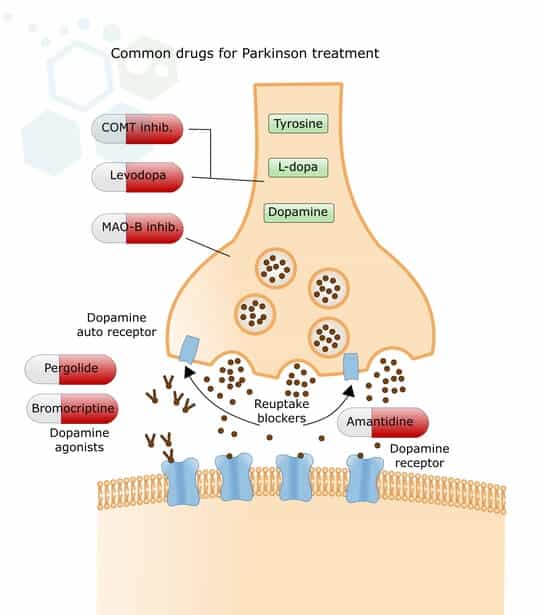
- Levodopa (L-Dopa) – Levodopa medication is a dopamine precursor and assists nerve cells in absorbing the medication and converting it into dopamine. L-Dopa has positive effects on mobility, muscle stiffness, and tremors. However, after a long period of L-Dopa therapy, results become to cause a variety of side effects, including hyperkinesia (unpredictable and uncontrollable movements) and on-off phenomena. To counteract fluctuating levels of efficacy in advanced stages of shaking palsy, some patients have a dopamine-producing pump installed internally that delivers the dopamine continuously via an implanted probe into the small intestine or under the skin.
- Carbidopa-levodopa (Sinemet)—This new medication combines carbidopa and levodopa. This more modern medication can prevent the destruction of levodopa in the digestive tract and help reduce undesired side effects, such as nausea.
- Dopamine Agonists – This group of PD medications mimics the effects of dopamine on brain cells, causing neuron cells to react to them precisely as they would to natural dopamine. These medications also increase the impact of the existing dopamine (pramipexole, lisuride, ropinirole). Newer forms of dopamine agonists ensure consistent even drug levels throughout the day. They must be taken in part only once daily (extended-release ropinirole, extended-release pramipexole) to increase dopamine levels. These medications can also deliver the active ingredient via a dermal patch, which must be changed daily (DescriptionRotigotine patch). Side effects of dopamine agonists may occur in the form of daytime fatigue or drowsiness and the form of compulsive behavior such as compulsive purchasing and gambling addictions.[4]
- Other groups of medications can help reduce the effects of dopamine (COMT inhibitors such as entacapone and tolcapone) or slow down its breakdown (MAO-B inhibitors such as selegiline and rasagiline).
- Anticholinergics (Biperiden, Bornaprin) can effectively reduce tremors and movement disorders.
- NMDA inhibitors amantadine can help to increase dopamine release and improve mobility for some patients with damage to substantia nigra pars
Is there a cure for Parkinson’s Disease?
For advanced Parkinson’s disease cases, some doctors also recommend using dopamine agonists and different groups of active ingredients with each other. Still, this approach is very challenging because each medication’s symptoms and side effects can differ, leading to an unexpected response to the combined medications. Current stem cell clinical trials have limitations, but stem cell research has shown promising results for halting disease progression. Isolated neural stem cells can differentiate into dopamine-producing neurons lost in Parkinson’s disease. Preliminary clinical trials used neurons from pluripotent stem cells; however, the researchers injected stem cell-derived neurons into the brains of Parkinson’s patients, resulting in complications. The Regeneration Center uses the latest stem cell technology and advances in stem cell manufacturing to contain the disease and improve motor function without the same risk as using IPSC cells. Our ongoing research is focused on the best methods to turn stem cells into neurons, the ideal type of stem cells to use, and the optimal delivery mechanisms. While there are still significant challenges to overcome, the future of stem cell therapies and neural stem cell therapies offer an exciting alternative treatment not just to manage symptoms but also to slow or stop the progression of Parkinson’s disease via dopamine cell replacement.
Deep Brain Stimulation (DBS) & Surgical Interventions for PD
For some patients with Parkinson’s who do not respond well to medication, they may be offered brain surgery as a treatment option. There are very few surgical options for treating conditions like MS & PD, but the most common one is called deep brain stimulation (DBS). DBS involves surgically implanting a device in the Brain that generates electrical impulses via a battery-operated neurostimulator. The results of DBS are not very effective over the long term and carry a lot of risk due to the need for performing surgery on a patient who is already sick. Due to this risk, doctors and patients must weigh the potential risks with benefits, and programmed pacemakers in the Brain should only be used when drug therapy is no longer helpful.
Stem Cell Treatment for Parkinson’s
In Parkinson’s disease, specific nerve cells die in the Brain. Clinical trials worldwide are, therefore, to find a new cure for Parkinson’s by replacing these dead neural stem cells with dopaminergic neurons, new functional cells, and neurotransmitters. One idea was to take stem cells from the bone marrow and implant them in the Brain (stem cell injection, stem cell transplantation). According to the theory, the stem cells in the patient’s mind should convert into new, functional nerve cells or at least compensate for deficits in people with Parkinson’s disease.
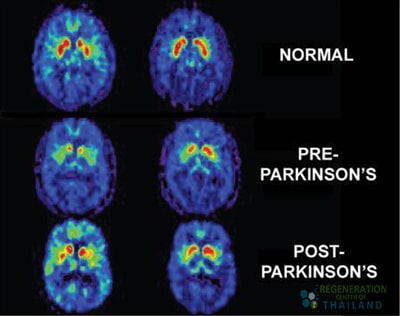
This approach sounded promising, but transplantation of bone marrow stem cell line did not always lead to the results wanted or ineffective and had potential harm to the patient. Since the development of adult stem cell therapy, new methods have been discovered to replicate/replace diseased cells using isolated and expanded mesenchymal cells ( UC-MSC+). Neural stem cells have proliferative activity in specific adult brain regions, particularly in the subventricular zone and hippocampus. These cells can differentiate into various neural cell types, including neurons and glia, but typically do not generate large numbers of dopamine neurons in the cortex.
Long-term effects of stem cell therapy for Parkinson’s Disease
We are still in the early stages of regenerative medicine, and the long-term effects of stem cell therapy for Parkinson’s disease are still largely unknown. Initial treatment results have shown some promising results, with patients experiencing an increase in dopamine cells and modest improvements in motor symptoms that persisted up to a couple of years after treatment. The actual test of stem cell therapies for Parkinson’s will be whether cell transplants can provide sustained dopamine production and related symptom relief over many years.
What are the side effects of stem cell therapy?
Longer-term safety and misuse of stem cell delivery methods also need to be carefully evaluated, as there are valid concerns about the types of cells used to have the potential for stem cell-derived grafts to eventually develop into tumors or cause other adverse effects later on. The Regeneration Center is committed to multi-year longitudinal studies to understand better the durability of improvements, optimal cell doses and delivery methods, and any delayed risks. Once proven safe and effective over the long run, stem cell therapy will modify the course of Parkinson’s, providing lasting regeneration of dopamine neurons and significantly improving quality of life.
Culturing Neurons & Neural Progenitor Cells
The dopamine-producing cells used in the treatment are first isolated from areas where neurogenesis occurs. The multipotent stem cells require culturing (expansion) over several days to differentiate them into functional astrocytes, neurons, and oligodendrocytes. The Regeneration Center’s propitiatory treatment protocol to treat movement disorders like Parkinson’s using neural stem cells is built on the latest advances in stem cell technologies and cell manufacturing.
Mesenchymal stem cells for Parkinson’s
The Regeneration Center uses isolated stem cells that have the potential to be unique as they target the central nervous system to enhance motor neuron function and improve overall quality of life. The therapy for people with Parkinson’s also focuses on the underlying mental symptoms via infusions of progenitor cells that can release dopa, which then raises the levels of new neuron cells needed to slow down, stop, or, in some cases, reverse the gradual progression of Parkinson’s before it’s too late.
Stem Cell Therapy for Memory Loss
The Regen Center Stem Cell Therapy for Parkinson’s allows us targeted and customizable dopamine-producing cell delivery to help induce neurogenesis over several stages, depending on the severity of the patient’s underlying health. The treatments are effective in being absorbed by the tissues after crossing the blood-brain barrier through cerebrospinal fluid or nebulizer.[5]
Treatment options for movement disorder & Parkinson’s’ using Stem cells w/ dopaminergic neurons may include:
- Radio Guided Therapy
- The stereotactic technique of deep brain stimulation
- Striatum stereotactic pallidotomy
- Subarachnoid stem cell implantation via lumbar puncture
- Brain cell stereotactic operation – Stem PD
Implanted neural stem cells help dopamine transport channels and eventually differentiate into dopaminergic neuron cells to continue as healthy & functional neurotransmitters. We use isolated Mesenchymal stem cells for treatment (UC-MSC+). These cells are differentiated into nerve cells, including dopamine-producing and dopamine agonists. The quantity & types of implanted cells (growth factors) depend strictly on the Parkinson’s disease patients’ medical needs.
Physiotherapy Management for Patients with Parkinson’s Disease
Physical therapy cannot reverse or stop Parkinson’s disease. Still, such treatment options can help improve a patient’s independence and overall quality of life by improving cardiovascular health, strength, movement range, and pain relief. Good physiotherapeutic care is essential to maintain mobility as long as possible. Speech therapy can also help patients when their language and swallowing are impaired. Non-verbal communication via gestures and facial expressions should also be encouraged whenever possible.
Occupational therapy and psychological care also help families and caretakers cope independently with everyday life or carry out hobbies as they would typically have. A professional therapist can help sufferers and families deal with the mental health problems or symptoms the patient might be experiencing.
Parkinson’s Diet: Foods to Eat and Avoid
While there is no single Parkinson-specific diet to maintain good health, most patients with PD should be more cautious than most by eliminating junk food and trying to maintain a balanced diet full of antioxidants (vegetables, fruits, and dark chocolate), whole grains, dairy fat, and protein-rich foods such as legumes and meat to boost dopamine levels. Lifestyle diets such as the Mediterranean diet and ketogenic diet can positively influence both motor and non-motor symptoms of patients with Parkinson’s, reduce systemic inflammation, and increase dopamine-producing cells in the body.
Stem Cell Treatment for Parkinson’s – 2025 Guidelines
The goal of stem cell therapy for Parkinson’s disease is to alleviate the patient’s symptoms and restore any lost neurological function. The treatment process is performed on an out-patient, and transplanting cells is done in a non-invasive way. Stem cell treatment for PD may not be a good option for all cases, so we request that potential patients submit recent medical diagnoses and lab results/CT scans to our medical team for review.
If you would like to learn more about how functional medical therapies can be applied in treating Parkinson’s Disease or have any other questions, please contact us today.
Published Clinical Citations
[1] ^ Agosta, Federica, Ammar Al-Chalabi, Massimo Filippi, Orla Hardiman, Ryuji Kaji, Vincent Meininger, Imaharu Nakano, et al. 2014. The El Escorial criteria: strengths and weaknesses. Amyotrophic lateral sclerosis & frontotemporal degeneration, no. 1-2 (December 8). doi:10.3109/21678421.2014.964258. https://www.ncbi.nlm.nih.gov/pubmed/25482030
[2] ^ Naujock, Maximilian, Nancy Stanslowsky, Peter Reinhardt, Jared Sterneckert, Alexandra Haase, Ulrich Martin, Kwang-Soo Kim, Reinhard Dengler, Florian Wegner, and Susanne Petri. 2014. Molecular and functional analyses of motor neurons generated from human cord-blood-derived induced pluripotent stem cells. Cure for Parkinson’s with Stem cells and development, no. 24 ( 15). doi:10.1089/scd.2014.0180. https://www.ncbi.nlm.nih.gov/pubmed/25007389
[3] ^ Phanthumchinda, K, O Supcharoen, and E Mitrabukdi. 1996. Madras pattern of motor neuron disease: case report from Thailand. Journal of the Medical Association of Thailand = Chotmaihet thangphaet, no. 6. https://www.ncbi.nlm.nih.gov/pubmed/8855616
[4] ^ Sarlak, Golmaryam, Anorut Jenwitheesuk, Banthit Chetsawang, and Piyarat Govitrapong. 2013. Effects of melatonin on nervous system aging: neurogenesis and neurodegeneration. Journal of Pharmacological Sciences, no. 1 (August 27). https://www.ncbi.nlm.nih.gov/pubmed/23985544
[5] ^ Terashima, Tomoya, Hideto Kojima, Hiroshi Urabe, Isamu Yamakawa, Nobuhiro Ogawa, Hiromichi Kawai, Lawrence Chan, and Hiroshi Maegawa. 2014. Stem cell factor-activated bone marrow ameliorates amyotrophic lateral sclerosis by promoting protective microglial migration. Journal of Neuroscience Research, no. 7. https://www.ncbi.nlm.nih.gov/pubmed/24936617