Neural stem cells have the capability to transform into glial cells or neurons. Oligodendrocytes used in treatment of TM and astrocytes are examples of glial cells.
What are Neural Cells and Why are they Important?
Different areas of the human body, spinal cord and brain house different types of specialized stem cells. In haematology for example, cord blood derived stem cells transplants have been used for decades for their abilty to replenish white blood cells and bone marrow cells for patients with a wide variety of blood disorders due to cancer. Similarly, neurogenerative conditions require special types of cells known as neural stem cells.
- Self-renewal potential
- Neural tripotency (ability to produce major neural lineages: astrocytes, neurons and oligodendrocytes)
- Ability for in vivo regeneration (post transplant)
The 5 types of Stem Cells
NSC cells have the potential to create both glia and neurons for human brains buy have limited regenerative capacity in a mature adult brain. For adults, neural cells reside in region called “neurogenic niches”. Their job is to retain multipotency and also to regulate balance between fate-committed asymmetric divisions and symmetrical self-renewal. After decades of research, new protocols and gene therapies have been developed for functional medical therapies using cultured neural cells to treat several diseases of the brain. From early onset such as Multiple sclerosis, cerebral palsy to late onset neurodegenerative diseases like Ataxia, Motor Neuron Disease, ALS, Alzheimer’s – Dementia, Parkinson’s disease (PD) cell manipulation technology has enabled the Regen Centre to isolate and expand human neural stem cells for use in therapies
Neural stem cells (NSCs) are a unique subset of stem cells found in the nervous system. Like all stem cells, NSCs have the ability to self-renew (make more stem cells) and to differentiate into more specialized cell types. In the context of the nervous system, this means that NSCs can differentiate into neurons, astrocytes, and oligodendrocytes.
Significance of Neural stem cells
- Locations in the Brain: NSCs were once thought to only exist during embryonic development. However, it’s now known that certain areas of the adult brain, such as the subventricular zone (SVZ) of the lateral ventricles and the dentate gyrus of the hippocampus, retain populations of NSCs.
- Role in Neurogenesis: One of the most fascinating discoveries in neurobiology was the recognition that neurogenesis, or the birth of new neurons, can occur in specific regions of the adult mammalian brain, especially the hippocampus. NSCs play a crucial role in this process.
- Potential for Repair: Given their capacity to differentiate into various neural cell types, NSCs hold potential for therapeutic use in neurodegenerative diseases, spinal cord injuries, and other conditions where neuronal damage occurs.
- Research and Clinical Implications: NSCs are of immense interest in the field of regenerative medicine. If scientists can harness and guide the differentiation of these cells, it may be possible to repair or replace damaged brain tissue.
- Challenges: While the potential of NSCs is vast, there are challenges to be addressed. These include the efficient and safe extraction, expansion, and implantation of these cells, as well as potential ethical considerations associated with certain sources of stem cells.
- Cancer Consideration: Like other stem cells, there’s a balance to be struck with NSCs. Their capacity for proliferation, which is beneficial for repair and regeneration, might also pose a risk. For instance, if not properly regulated, there’s a theoretical risk of tumor formation.
- Future Directions: As research progresses, there’s hope that NSCs could be utilized not just for direct therapeutic applications but also as a tool for drug discovery, modeling of neurological diseases, and more.
Neural stem cells benefits
Neural stem cells represent a promising frontier in neuroscience and regenerative medicine. The challenge lies in translating the potential of these cells into safe and effective therapies for a host of neurological disorders. We are not currently at the stage where we can cure any/all neurodegenerative diseases so the goal of our NSC treatments is to first stop the progress of the disease and then replace and/or repair diseased or necrotic (dead) cells. Unlike other stem cell technologies that try to use just hematopoietic mesenchymal cells for brain injuries our treatment protocols used transplanted neural cells, neural progenitor cells and neural cell specific growth factors to ensure that any new transplanted cells survive after transplants and are able to integrate into the patients tissue. Any implanted cells for brain injuries or strokes must not only look like upper motor neurons but must also have vital properties that allow them to function as neurons. The cells used must be able to release neurotransmitters, be electrically excitable and create new circuitry / neural structures for complex brain processes and neural synapses.
Currently, conventional pharmacological based treatments for neurodegenerative diseases can help relieve some of the patients symptoms but usually do not alter the course of the underlying disease nor halt its progression.
Therapeutic Plasticity from Neural Stem Cells
Neuroplasticity, often called brain plasticity or neural plasticity, is a fundamental brain property that allows it to change and adapt in response to new experiences, learning, and environmental changes. This adaptability is crucial for brain development, education, memory formation, brain injury recovery, and sensory loss adaptation. Neuroplastic changes can occur at various levels, from cellular changes involving the strengthening or weakening of synapses (the connections between neurons) to large-scale cortical remapping, where the function of one brain area can be transferred to another area following injury or sensory deprivation.
Therapeutic plasticity from neural stem cells (NSCs) represents a cutting-edge area of research that aims to harness the inherent regenerative capacities of NSCs to repair and rejuvenate the nervous system. NSCs can self-renew and differentiate into various neural cell types, offering potential treatment strategies for neurodegenerative diseases, spinal cord injuries (SCI), traumatic brain injuries (TBI), and other neurological conditions.
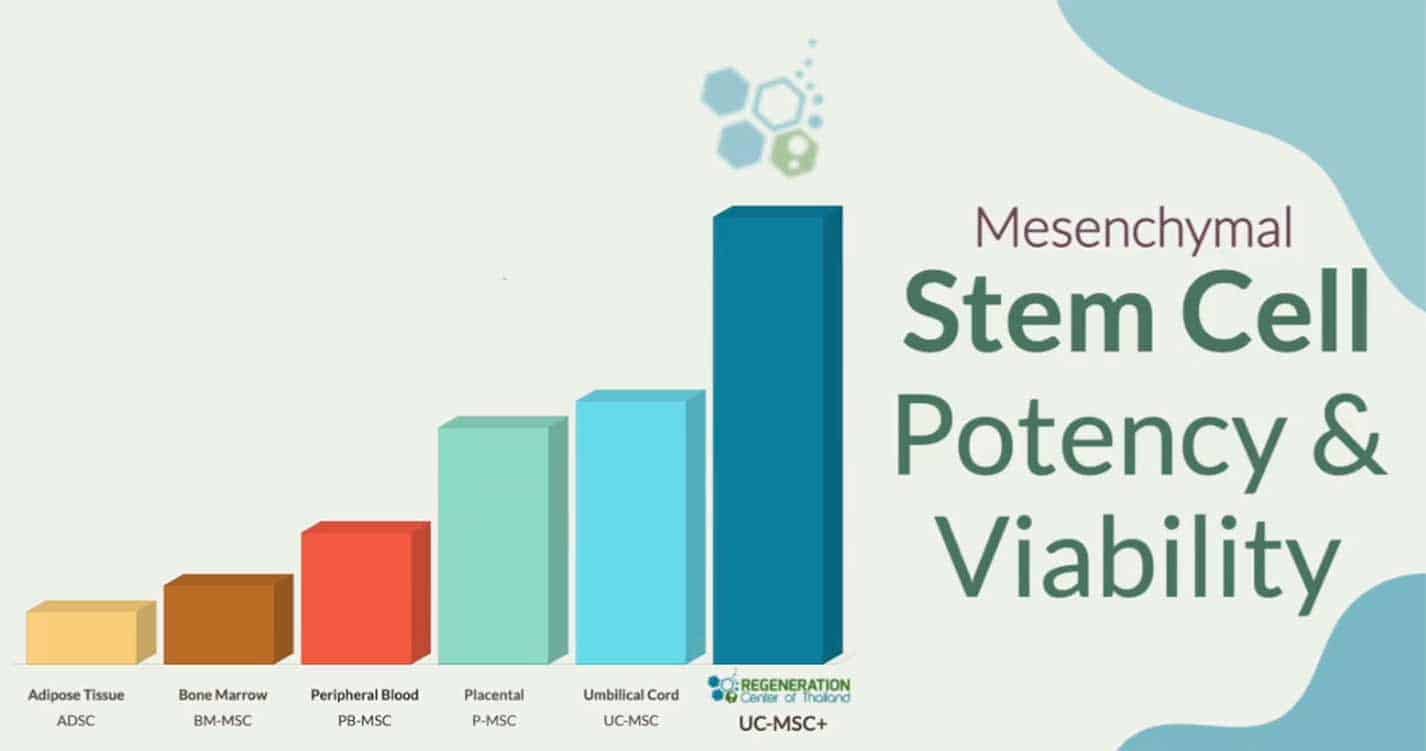
Mesenchymal stem cells’ (MSCs) secretome, including their released proteins, lipids, and nucleic acids, can support neural regeneration and provide neuroprotection. In spinal cord injury cases, where lesions lead to the loss of neurons and glial cells, causing inflammation, demyelination, and pain, the MSC-derived secretome has demonstrated a neuroprotective role. It has been shown to promote the outgrowth of axons through the action of trophic factors such as brain-derived neurotrophic factor (BDNF), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF). These factors not only support the survival and growth of neural cells but also lead to the reduction of damage induced by oxygen-glucose deprivation and enhance the formation of neuronal connections.
Furthermore, intrathecal administration of MSC stem cells can have direct neuroprotective and neurodegenerative effects in experimental models of spinal cord injury. These exosomes can modulate the microenvironment of spinal cord lesions by delivering anti-inflammatory and pro-angiogenic factors, thereby suppressing inflammation, reducing the size of the lesion, and supporting functional recovery. The Regeneration Centers’ therapeutic approach offers a promising avenue for treating various neurodegenerative diseases and injuries by leveraging the body’s innate repair mechanisms.