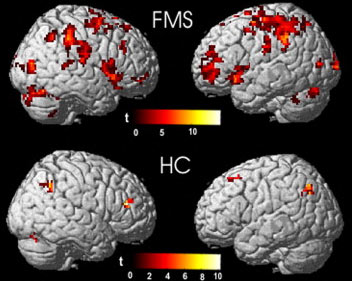
Fibromyalgia Syndrome is also known as FM or FMS. Fibromyalgia is technically classified as a musculoskeletal disorder, but the condition is now 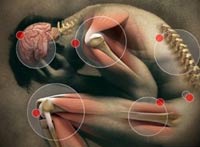 also being recognized as an issue relating to the central nervous system also. Symptoms for FM include stiff painful joints, chronic fatigue, increased sensitivity to pain. It also manifests symptoms on specific points on a person chest, arms, legs or back. Other common symptoms include random migraines, irritable bowel syndrome and sleep disorders are often the common theme amongst those suffering from Fibromyalgia. It is estimated that nearly 4% percent of the world’s population may be suffering from fibromyalgia syndrome, but even with all our advancements in medicine, there is still no clear cause for the syndrome and the condition is sometimes very difficult to diagnose clinically.
also being recognized as an issue relating to the central nervous system also. Symptoms for FM include stiff painful joints, chronic fatigue, increased sensitivity to pain. It also manifests symptoms on specific points on a person chest, arms, legs or back. Other common symptoms include random migraines, irritable bowel syndrome and sleep disorders are often the common theme amongst those suffering from Fibromyalgia. It is estimated that nearly 4% percent of the world’s population may be suffering from fibromyalgia syndrome, but even with all our advancements in medicine, there is still no clear cause for the syndrome and the condition is sometimes very difficult to diagnose clinically.
Symptoms of Fibromyalgia
The intensity of the usual fibromyalgia symptoms will differ from one individual to the next and might change based on other aspects such as time of day or the weather conditions. Due to the fact that FMS is a persistent condition, the majority of fibromyalgia symptoms may never vanish unless treated.
fibromyalgia clinical trials and research have shown that most victims display having pain in certain areas, while others might experience total body discomfort including pain in muscles and tendons similar to some patients with multiple sclerosis.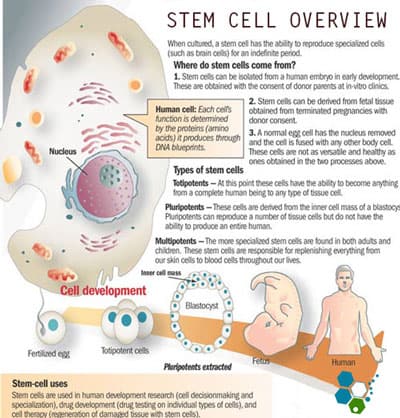
Other symptoms of Fibromyalgia Include:
- Memory troubles.
- Sleep disruptions
- Sleep apnea
- Bruxism (teeth grinding)
- Severe Pain in the Knees
- Irritable bowel syndrome (IBS)
- Late-onset MG
- Lyme disease & CVID
- Systemic lupus erythematosus
- Inflammatory bowel diseases such as ulcerative colitis and crohn’s disease – IBD
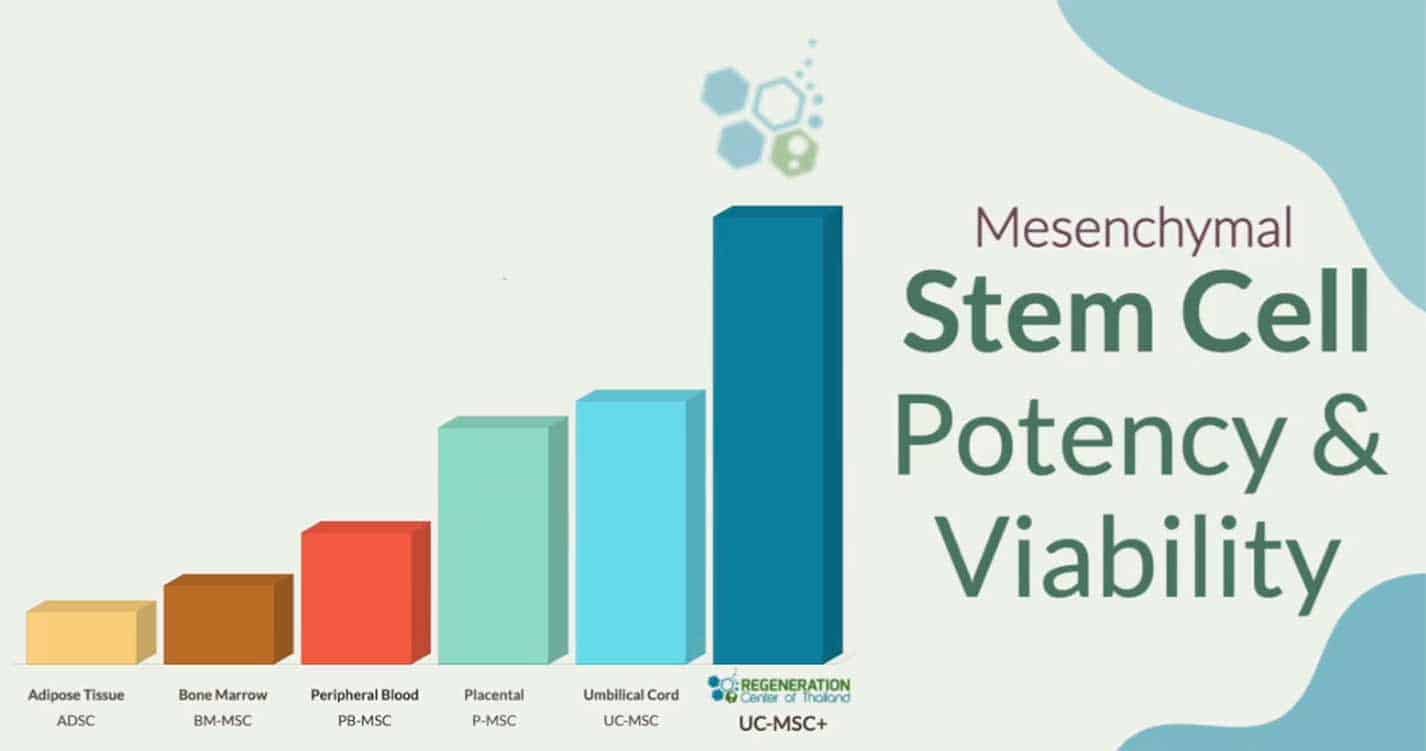
Treatment Fibromyalgia with MSC+ Stem Cells
Stem cells are considered the building blocks of life due to their amazing creative and natural tendency to regenerate our bodies in case of injuries. They are essentially the bodies pharmacy.
 The Regeneration center offers an effective treatment protocol for Fibromyalgia and Chronic Fatigue. Our treatment is unique because our protocol introduces a series of multi-stage therapeutic injections of CD34+ Mesenchymal stem cells to the patient’s body for a very targeted regeneration therapy. Similar multi-stage treatments are also used for movement disorders, Cerebral Palsy, Diabetes type 2, heart disease and lung disease such as Idiopathic Pulmonary Fibrosis.
The Regeneration center offers an effective treatment protocol for Fibromyalgia and Chronic Fatigue. Our treatment is unique because our protocol introduces a series of multi-stage therapeutic injections of CD34+ Mesenchymal stem cells to the patient’s body for a very targeted regeneration therapy. Similar multi-stage treatments are also used for movement disorders, Cerebral Palsy, Diabetes type 2, heart disease and lung disease such as Idiopathic Pulmonary Fibrosis.
In times of distress, our bodies release a naturally occurring protein called SDF-1. This protein guides the exact movement of other cells surrounding cells to begin homing and help begin the healing process. This “homing” function helps the newly introduced stem cells in your body to travel to the appropriate areas for repair duties. Autologous (From Your Body) or Allogeneic (HLA Matched and Donated) stem cells are typically isolated from peripheral blood, cord tissue, placental tissue, bone marrow or adipose fat.[1]
The cells are then processed and expanded in our single system GMP approved Class 5 Biomedical clean rooms where our microbiologists are able to prepare your therapeutic doses of stem cells.[2]
The prepared MSC enriched stem cells and neurogenic growth factors are reinjected in multiple stages to promote rapid healing of the previously damaged cells.
TREATMENT PRECAUTIONS & RISKS
Fibromyalgics may have many different symptoms and types of pain in multiple areas of concerns. To qualify for our treatment all patients must be clinically diagnosed in his or her home country first using standard Blood and urine tests. Clinical diagnosis typically requires 3 months of widespread pain. Our doctors will need to review your previous lab results, DNA tests or diagnosis before determining eligibility to travel to Thailand for treatment. Patients with other medical conditions such as PKD, recent heart attack will not qualify. Learn more about brain friendly dietsThe ability to extract and properly expand/grow stem cells for an extended period of time is unique in the treatments we provide. Expansion or lab growing of stem cells is currently prohibited in some countries.[3]
FMS & Chronic Fatigue Treatment Guidelines in 2024
Total Number of Stem Cell Infusions for FMS and CF will vary based on Patient needs.
Types of Cells Used for in Fibromyalgia Treatment Protocol: Enriched Hematopoietic MSC+ Stem cells do not require any invasive surgeries to harvest cells and the staged cell infusions are usually made via intravenous Drips, Intrathecal Injections or radio-guided injections (when necessary). [4]
Post Treatment Rehab – Optional: Physical Rehabilitation is typically not required unless there are other underlying physical issues. with the patients. We do however offer complete physical rehabilitation services post-therapy in Bangkok as Optional. Our rehab team can assist you upon request for 2-5 hours per day and up to 6 days per week. Medical travel visas assistance and extended stay accommodations at a hotel or extended stay apartment for the patient and family can also be provided upon request.
Functional Healthcare – The Medicine of Connectivity
Treating Fibromyalgia with MSC+ Stem Cells
Our multi-stage treatment for Fibromyalgia with Stem Cells will require 2-3 weeks in Bangkok depending on the patients’ needs. Due to the varying degrees, areas, and sources of injuries, our rheumatologist and medical team will need to better understand a potential patient before a detailed treatment protocol can be provided. Upon approval, a detailed treatment plan will be provided that will include the specifics such as day by day calendar with stages, exact total number nights required along with the total medical related costs (excluding accommodations or flights). To begin the qualification process for our multi-stage treatment protocol please prepare your recent medical records such as blood and urine tests and contact us today.
Published Clinical Citations
[1] ^ Yeephu, Suwimon, Chuthamanee Suthisisang, Saithip Suttiruksa, Pradit Prateepavanich, Patchara Limampai, and Irwin Jon Russell. 2013. Efficacy and safety of mirtazapine in fibromyalgia syndrome patients: a randomized placebo-controlled pilot study in Thailand. The Annals of pharmacotherapy, no. 7-8 (June 4). doi:10.1345/aph.1R725. https://www.ncbi.nlm.nih.gov/pubmed/23737510
[2] ^ Gunduz, B, Y A Bayazit, F Celenk, C Saridoğan, A G Guclu, E Orcan, and J Meray. 2008. Absence of contralateral suppression of transiently evoked otoacoustic emissions in fibromyalgia syndrome. The Journal of laryngology and otology, no. 10 (March 4). doi:10.1017/S0022215107001569. https://www.ncbi.nlm.nih.gov/pubmed/18318918
[3] ^ Busse, Jason W, Shanil Ebrahim, Gaelan Connell, Eric A Coomes, Paul Bruno, Keshena Malik, David Torrance, et al. 2013. Systematic review and network meta-analysis of interventions for fibromyalgia: a protocol. Systematic reviews (March 13). doi:10.1186/2046-4053-2-18. https://www.ncbi.nlm.nih.gov/pubmed/23497523
[4] ^ Üçeyler, Nurcan, Daniel Zeller, Ann-Kathrin Kahn, Susanne Kewenig, Sarah Kittel-Schneider, Annina Schmid, Jordi Casanova-Molla, Karlheinz Reiners, and Claudia Sommer. 2013. Small fibre pathology in patients with fibromyalgia syndrome. Brain : a journal of neurology, no. Pt 6 (March 9). doi:10.1093/brain/awt053. https://www.ncbi.nlm.nih.gov/pubmed/23474848