New research shows that specific types of brain cells become active after brain injuries and exhibit properties similar to those of neural stem cells. Astrocyte plasticity might correlate with the upregulation of the Galectin 3 protein, which may significantly contribute to discovery of additional biomarkers. The study discovered that a specific protein regulates these cells and could be a target for therapy and contribute to development of better treatments options for brain injuries. The loss of neurons, which subsequently causes impairment of brain function, is caused by the onset and progression of neurological disorders, like strokes, spinal cord injuries and neurodegenerative diseases such as Parkinson’s, Alzheimers / Dementia, ALS and MND. Effective treatment options still need to be improved. However, preclinical research has shown a promising response involving reactive astrocytes, a specific type of glial cell, which is a crucial part of the nervous system alongside neurons. Microglia and Glial cells are regarded as a safeguard for neurons, demonstrating the ability to resume cell proliferation, a mechanism essential for protecting the injury-affected brain from invasion by immune cells.[1]
Differentiation of Mesenchymal Stem Cells to Neuroglia
What are Astrocytes?
Astrocytes are a type of glial cell in the brain and spinal cord. They are the most abundant cell types in the human brain and play a crucial role in the functioning of the nervous system [2]. Here are some critical aspects of astrocytes:
- Support and Structure: Astrocytes give structural support to neurons, promote neurogenesis and maintain the environment in which neurons operate.
- Blood-Brain Barrier: They are involved in forming and maintaining the blood-brain barrier, which controls the movement of substances between the bloodstream and the brain.
- Nutrient and Waste Management: Astrocytes regulate the concentration of ions and neurotransmitters in the extracellular space. They also help in nutrient transport to neurons and waste removal.
- Repair and Protective Scarring: Astrocytes can proliferate and form a scar (physical and biochemical barrier) around the injured area. This barrier helps to contain the damage and prevent it from spreading to healthy brain tissue. This is part of the brain’s normal repair process, following injury to the nervous system.
- Neurotransmitter Regulation: They can modulate neurotransmission by absorbing and releasing neurotransmitters and via astrocytes apoptosis.
- Neuron Health: Astrocytes function are essential for the survival and functionality of neurons, aiding in repairing, releasing neutrophils and protecting nervous system tissue.
- Neural Plasticity: They play a role in the formation and remodelling of synapses, influencing learning and memory.
Astrocytes exhibit a star-like shape, hence the name “astro” (star in Greek) and “cyte” (cell). They are increasingly recognized for their diverse and dynamic roles in the central nervous system beyond their traditional supportive functions [3].
Role of Astrocytes vs Oligodendrocytes
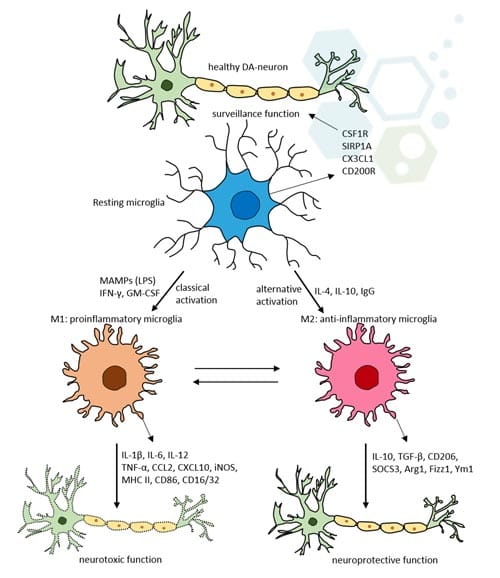 Astrocytes and oligodendrocytes are two vital types of glial cells in the central nervous system, each playing unique and critical roles. Astrocytes, characterized by their star-shaped structure, are primarily responsible for providing structural support to neurons. They play a crucial role in maintaining the blood-brain barrier, which controls the movement of substances from the bloodstream into the brain. Additionally, astrocytes facilitate the transport of nutrients to neurons, regulate and recycle neurotransmitters, and are involved in the repair and scarring processes in the brain following injuries. They also help maintain the ionic and chemical environment essential for neuronal function along with glial cells astrocytes & optic nerve astrocytes. In contrast, oligodendrocytes, which are smaller and have fewer processes than human astrocytes, are primarily known for their role in myelination. They produce myelin, a fatty substance that forms a sheath around the axons of neurons in the central nervous system. This myelination is crucial for the rapid transmission of electrical signals along neurons, enhancing the speed and efficiency of neurotransmission. Oligodendrocytes support axons and are essential for their maintenance within the central nervous system. Both astrocyte cell culture and oligodendrocytes are indispensable for properly functioning the nervous system. While astrocytes are more engaged in creating and maintaining the neuronal environment, oligodendrocytes focus on facilitating rapid signal transmission. Their complementary functions ensure that the nervous system operates efficiently, highlighting the complexity and sophistication of neural processes.
Astrocytes and oligodendrocytes are two vital types of glial cells in the central nervous system, each playing unique and critical roles. Astrocytes, characterized by their star-shaped structure, are primarily responsible for providing structural support to neurons. They play a crucial role in maintaining the blood-brain barrier, which controls the movement of substances from the bloodstream into the brain. Additionally, astrocytes facilitate the transport of nutrients to neurons, regulate and recycle neurotransmitters, and are involved in the repair and scarring processes in the brain following injuries. They also help maintain the ionic and chemical environment essential for neuronal function along with glial cells astrocytes & optic nerve astrocytes. In contrast, oligodendrocytes, which are smaller and have fewer processes than human astrocytes, are primarily known for their role in myelination. They produce myelin, a fatty substance that forms a sheath around the axons of neurons in the central nervous system. This myelination is crucial for the rapid transmission of electrical signals along neurons, enhancing the speed and efficiency of neurotransmission. Oligodendrocytes support axons and are essential for their maintenance within the central nervous system. Both astrocyte cell culture and oligodendrocytes are indispensable for properly functioning the nervous system. While astrocytes are more engaged in creating and maintaining the neuronal environment, oligodendrocytes focus on facilitating rapid signal transmission. Their complementary functions ensure that the nervous system operates efficiently, highlighting the complexity and sophistication of neural processes.
Can Astrocytes gain neural stemness properties?
Astrocytes can be re-expressed to gain neural stem cell (NSC) properties and have the potential for brain repair and regeneration. Astrocytes can gain or exhibit neural stem cell properties in several ways, including:
- Injury-Induced Reactivation: In response to a brain injury or disease, astrocytes can revert to a more primitive, stem cell-like state. This process, known as reactive gliosis, often sees astrocytes re-expressing genes typically active in NSC during astrocyte phagocytosis
- Molecular and Environmental Cues: Research at the Regeneration Center has shown that specific molecular and environmental cues induce astrocytes to differentiate into a stem-cell-like state. For example, introducing certain transcription factors or exposure to particular growth factors can trigger this transformation.
- Epigenetic Changes: Changes in the epigenetic landscape of astrocytes, such as alterations in DNA methylation and histone modifications, can reprogram them to acquire stem cell-like properties.
- Gene Editing Techniques: Advanced genetic manipulation techniques like CRISPR/Cas9 can be used to modify the gene expression of astrocytes, potentially reprogramming them into NSCs.
- Cell Fusion Events: In rare instances, cell fusion events in the brain might lead to the merger of an astrocyte with a neural stem cell, potentially endowing the astrocyte with Neural Stem Cell characteristics.
- Microenvironmental Influences: The brain’s microenvironment, including specific extracellular matrix components and neighbouring cells, can influence astrocyte behaviour and potentially promote stem cell-like properties.
- Neurogenic Niches: Some types of Astrocytes in some brain areas, like the subventricular zone (SVZ) and hippocampus, are already in a more plastic state and closer to NSCs regarding their potential to form new neurons.
- Stress Response: Under certain stress conditions, astrocytes exosomes may revert to a more plastic, stem-cell-like state as part of the brain’s intrinsic repair mechanism.
These insights into astrocyte plasticity and their potential to gain NSC properties are crucial for The Regeneration Center when developing new therapeutic strategies for neurodegenerative diseases and other neurological conditions like Ataxia, MS, transverse myelitis and peripheral neuropathy. However, our results are preliminary and more studies need to be done to fully understand and safely harness this capability for clinical applications [4].
What do Astrocytes do?
Some of these astrocytes gain neural stem cell-like properties to self-renew and produce different cell types, including neurons and glial cells. Once a disease or injury-related disruption of the 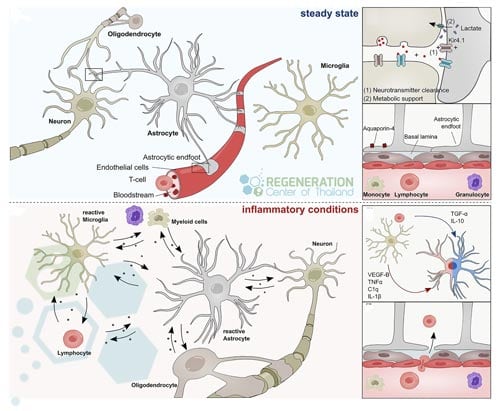 blood-brain barrier (BBB) happens, astrocyte markers and neural cell growth factors can initiate, increase and gain neural stem cell properties. Research suggests that pathology-specific astrocyte plasticity at a brain injury site can be correlated with the upregulation of the Galectin 3 protein.
blood-brain barrier (BBB) happens, astrocyte markers and neural cell growth factors can initiate, increase and gain neural stem cell properties. Research suggests that pathology-specific astrocyte plasticity at a brain injury site can be correlated with the upregulation of the Galectin 3 protein.
Galectin 3 Protein Biomarker
Galectin-3 is a type of protein belonging to the lectin family, which are proteins known for binding to specific carbohydrate structures. Some useful aspects of Galectin-3 include:
- Structure and Binding: Galectin-3 has a carbohydrate recognition domain (CRD) that allows it to bind specifically to beta-galactoside sugars. This binding capability is crucial for its role in various cellular processes.
- Cellular Functions: It is involved in a wide range of cellular functions, including cell growth, adhesion, differentiation, angiogenesis (formation of new blood vessels), apoptosis (programmed cell death), and inflammation.
- Role in the Immune System: Galectin-3 plays a significant role in the immune system. It modulates immune responses, including the activation and maturation of immune cells and inflammation processes.
- Cancer Research: It has gained attention in cancer research due to its high expression in many types of cancers and its role in tumour progression, metastasis, and angiogenesis. Galectin-3 is considered a potential biomarker for cancer diagnosis and a target for therapy.
- Fibrosis: Galectin-3 is implicated in developing fibrosis in various organs, such as the liver, kidney, and heart. It mediates the activation of fibroblasts and the accumulation of extracellular matrix components.
- Heart Disease: Elevated levels of Galectin-3 are associated with congestive heart failure and heart attacks and can be a marker for the diagnosis and prognosis of this condition.
- Infectious Diseases: It plays a role in host-pathogen interactions, influencing the body’s response to infections by various bacteria, viruses, and fungi.
- Cell Adhesion and Migration: Galectin-3 is involved in cell adhesion processes and can influence cell homing, cell migration, essential in wound healing and cancer metastasis.
- Neurobiology: There is emerging research on the role of Galectin-3 in neurobiology, particularly in neuroinflammation and neurodegenerative diseases.
Astrocytes for neurodegenerative diseases
Given the importance of astrocyte proliferation, these findings are relevant for understanding how changes in cerebrospinal fluid composition (upregulation of Galectin 3 protein) support the maintenance of astrocyte plasticity in the brain. Identifying Galectin 3 protein as an inducer of astrocyte plasticity has helped discover other biomarkers that offer beneficial modulation inside the injured brain parenchyma. These regulators of astrocyte proliferation after acute injury offer great promise for the future clinical applications of these biomarkers as indicators for detecting a beneficial reaction of glial stem cell therapy or help identify the presence of other cells with stemness potential in an injured patient’s brain [5].
Published Clinical Citations
[1] ^ Rauf A, Badoni H, Abu-Izneid T, Olatunde A, Rahman MM, Painuli S, Semwal P, Wilairatana P, Mubarak MS. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules. 2022 May 17;27(10):3194. doi: 10.3390/molecules27103194. PMID: 35630670; PMCID: PMC9146652.
[2] ^Dejakaisaya H, Kwan P, Jones NC. Astrocyte and glutamate involvement in the pathogenesis of epilepsy in Alzheimer’s disease. Epilepsia. 2021 Jul;62(7):1485-1493. doi: 10.1111/epi.16918. Epub 2021 May 10. PMID: 33971019.
[3] ^ Freeman MR. Specification and morphogenesis of astrocytes. Science. 2010 Nov 5;330(6005):774-8. doi: 10.1126/science.1190928. PMID: 21051628; PMCID: PMC5201129.
[4] ^ Ganapathy K, Datta I, Bhonde R. Astrocyte-Like Cells Differentiated from Dental Pulp Stem Cells Protect Dopaminergic Neurons Against 6-Hydroxydopamine Toxicity. Mol Neurobiol. 2019 Jun;56(6):4395-4413. doi: 10.1007/s12035-018-1367-3. Epub 2018 Oct 16. PMID: 30327976.
[5] ^ Alisch M, Kerkering J, Crowley T, Rosiewicz K, Paul F, Siffrin V. Identification of the gliogenic state of human neural stem cells to optimize in vitro astrocyte differentiation. J Neurosci Methods. 2021 Sep 1;361:109284. doi: 10.1016/j.jneumeth.2021.109284. Epub 2021 Jul 7. PMID: 34242705.

