Zombie cells, also called senescent cells, are non-dividing cells that accumulate in the body due to stress or damage, resisting normal apoptotic pathways. These cells contribute to aging and various diseases by secreting pro-inflammatory cytokines and growth factors, known as the SASP, which disrupts tissue function and promotes chronic inflammation. Advances in clinical research and stem cell therapies targeting removing these dysfunctional cells are being explored to mitigate their adverse effects on health. Senolytics aim to rejuvenate affected tissues by clearing senescent cells, enhancing overall health, and potentially extending lifespan. Harnessing the full potential of these new therapies opens up many new possibilities in functional healthcare and regenerative medicine.
What Are Zombie Cells
Zombie cells, scientifically known as senescent cells, are cells that have ceased dividing but persist in the body, contributing to aging and various metabolic diseases. These cells, originating from normal cells that have encountered stress or damage, undergo a complex metamorphosis instead of dying. This metamorphosis leads them to resist apoptosis, the body’s usual method of eliminating dysfunctional cells. By dodging this programmed cell death, zombie cells accumulate, adversely affecting their surroundings.
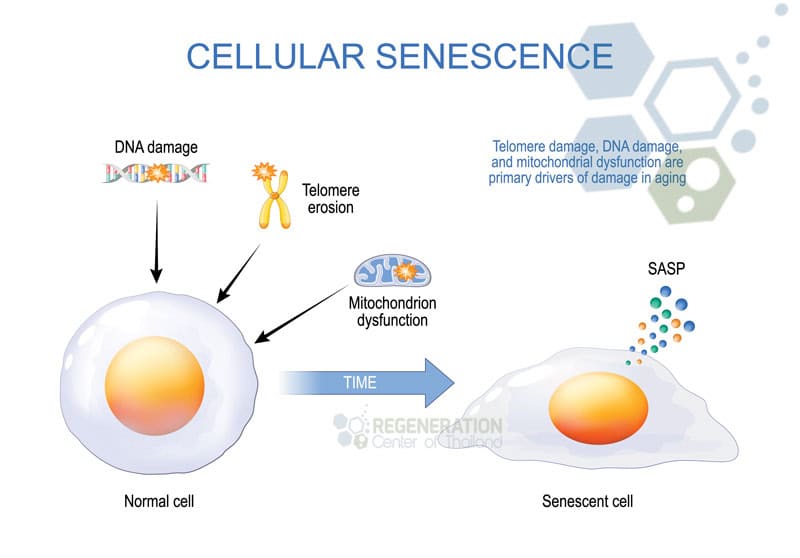
The resistance to cell apoptosis is an essential aspect of zombie cell origins. This resistance allows these cells to evade the immune system’s efforts to clear them out, leading to immune evasion. Such evasion is problematic because it prevents the immune system from maintaining cellular homeostasis, thus allowing these senescent cells to accumulate further.
As they accumulate, zombie cells are not merely passive occupants in the body. They actively induce inflammation through the secretion of pro-inflammatory cytokines and chemokines, a phenomenon known as the senescence-associated secretory phenotype (SASP) [1]. This inflammation can lead to various pathologies, exacerbating the aging process and contributing to the development of age-related diseases.
Moreover, these cells contribute to protein aggregation, linked with diseases like Alzheimer’s, ALS, MND, Ataxia, multiple sclerosis, transverse myelitis and Parkinson’s. Proteins, which usually fold into specific structures to perform cellular functions, begin to misfold and accumulate, disrupting cellular function and health.
Understanding zombie cells — from their origins and resistance mechanisms to their effects like inflammation induction and protein aggregation — is significant. This knowledge deepens our understanding and paves the way for therapeutic strategies that the Regeneration Center can use to ameliorate their negative impact on our health.
The Role of Senescent Cells
Senescent cells play a multifaceted role in the aging process and the progression of various age-related diseases. As cells that no longer divide but refuse to undergo apoptosis, they accumulate in tissues, contributing significantly to their dysfunction. This accumulation can disrupt the intricate cellular communication necessary for maintaining healthy tissue architecture and function. These cells do not exist in isolation; they interact dynamically within the cellular community, impacting neighboring cells and the overall microenvironment.[2]
Vital to understanding the detrimental effects of senescent cells is their link to inflammation. They secrete various pro-inflammatory cytokines, growth factors, and proteases, collectively known as the senescence-associated secretory phenotype (SASP). This secretion can initiate an inflammation response that, while initially beneficial for tissue repair, becomes chronic and harmful with persistent senescence. Chronic inflammation is a known contributor to many conditions such as spinal cord injuries, spinal stenosis, DDD, crohn’s , renal failure, liver cirrhosis, COPD and pulmonary fibrosis.
Senescent cells should be cleared by the immune system, a process known as immune clearance [3]. However, as we age, the efficiency of this system declines, allowing more senescent cells to accumulate and exacerbate tissue dysfunction. This dysfunction hinders tissue repair and regeneration and promotes the development of age-related pathologies.
Understanding senescent cell dynamics and their removal through targeted therapies offers a promising pathway to mitigate aging effects and extend health span. By enhancing senescent cell apoptosis— their programmed cell death—and improving their clearance from the body, we might rejuvenate tissues and delay the onset of age-related diseases, nurturing a sense of rejuvenation and belonging in our aging population.
Causes of Cellular Senescence
Various factors, including genetic mutations, telomere shortening, oxidative stress, and exposure to certain chemicals can trigger cellular senescence. As we explore these complex triggers, it’s essential to contemplate how each factor contributes to the broader community of our body’s cells, influencing their path toward senescence.
Genetic mutations often initiate the DNA damage response, a vital mechanism that prevents the proliferation of potentially cancerous cells by driving them into senescence. This response is essential in maintaining the integrity of our cellular population, ensuring that damaged cells do not replicate unchecked. However, when the DNA repair mechanisms are overwhelmed or compromised, the result can be the accumulation of senescent cells.
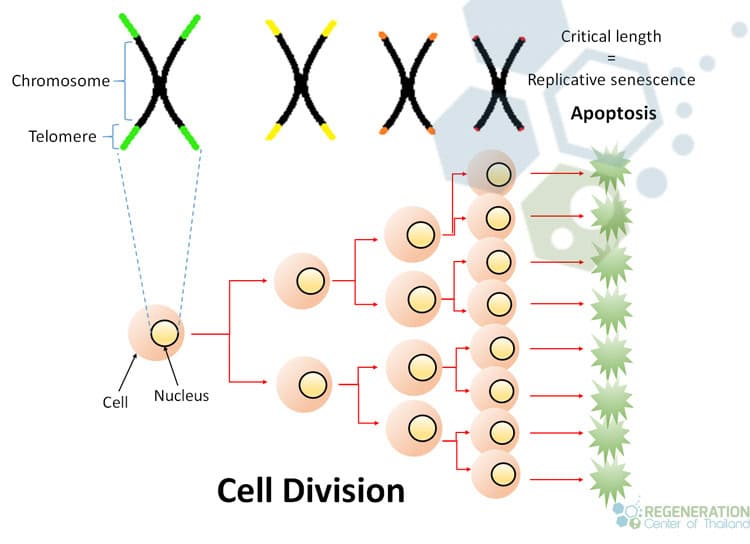
Telomere attrition, or the gradual shortening of telomeres, also plays a pivotal role in cellular aging. Telomeres protect the ends of chromosomes; as cells divide, these protective caps wear down. When they become too short, the cell can no longer divide safely, triggering senescence. This natural decline is a shared fate among all proliferating cells, highlighting a collective aspect of cellular aging.
Oxidative stress impact is another critical factor. Reactive oxygen species (ROS) can damage cellular components, leading to dysfunction and senescence. This oxidative stress is not only a result of internal metabolic processes but can also be exacerbated by external environmental factors, linking our cells to a web of shared environmental influences.
Additionally, oncogene activation and chronic inflammation are significant contributors. Oncogenes, when activated, can push cells toward a proliferative state that promotes senescence when combined with other stresses. Chronic inflammation creates a hostile environment for cells, promoting senescence through the persistent release of inflammatory cytokines and other mediators.
Understanding these causes helps us appreciate the interconnectedness of cellular behavior, drawing us into a more profound sense of community with our biological processes.
Zombie Cells and Aging
 Many cells in the aging human body change into a state known as senescence, where they cease to divide and can contribute to various age-related diseases. This change, often called the accumulation of ‘zombie cells,’ plays a crucial role in the aging process and how our bodies function as we age.
Many cells in the aging human body change into a state known as senescence, where they cease to divide and can contribute to various age-related diseases. This change, often called the accumulation of ‘zombie cells,’ plays a crucial role in the aging process and how our bodies function as we age.
The passage of aging is one we all share, a universal aspect of life that unites us. Within this progression, understanding the role of cellular senescence offers us a glimpse into why our bodies change over time and how we might influence these changes. Key factors such as cellular detoxification and antioxidant benefits play pivotal roles. By enhancing cellular detoxification, we can potentially reduce the burden of damaged cells, and with the strategic use of antioxidants, we might mitigate the harmful effects of free radicals, thereby slowing the aging process.
Furthermore, lifestyle choices significantly impact the accumulation and effects of senescent cells. Dietary influences can either accelerate or decelerate cellular aging. Diets rich in anti-inflammatory components and low in processed foods may support cellular health and longevity. Similarly, the impact of exercise in promoting blood flow and enhancing cellular repair mechanisms can also be instrumental in managing senescent cell accumulation.
Lastly, genetic predispositions cannot be overlooked. While we share the aging progression, our paths can vary significantly due to genetic differences. These predispositions can dictate how quickly senescent cells accumulate and how our bodies respond to internal and external interventions such as stem cell therapy to manage aging.
By understanding these aspects, The Regeneration Center can better traverse treatment limitations due to the complexities of aging, nurturing a sense of belonging in our collective quest for longevity and health.
Identifying Zombie Cells
Identifying zombie cells involves precise scientific techniques that detect markers indicative of cellular senescence. These cellular markers are crucial to understanding how cells shift into a senescent state, often called ‘zombie’ due to their inability to divide but continued metabolic activity. Sophisticated detection techniques are essential in pinpointing these markers, enabling us to map the presence and impact of senescent cells in tissue.
Tissue biopsy procedures play a vital role in this identification process. By extracting small tissue samples, we can utilize various laboratory tools to analyze these samples for signs of senescence. This direct approach allows for an in-depth examination of cellular behavior and the accumulation of senescent cells within various organs. Furthermore, advancements in imaging techniques have greatly enhanced the precision with which these cells can be observed. Modern imaging technologies provide a clearer picture of the cellular environment and facilitate a non-invasive peek into the body’s internal workings, making the detection of senescent cells less cumbersome and more accessible for most patients.
As we utilize these scientific capabilities, from biopsy procedures to cutting-edge imaging and laboratory tools, it propels our abilities to heal forward, enhancing our understanding of cellular senescence and its implications for health and longevity. By refining these detection methodologies, we edge closer to potentially mitigating the effects of aging and associated disorders, ensuring a collaborative pursuit of wellness and vitality.
The Emergence of Senolytics
Building on the identification of senescent cells, researchers have developed a new class of medications known as senolytics, which specifically target and eliminate these dysfunctional cells. This breakthrough offers hope for treatments that could rejuvenate tissues and extend a healthy lifespan. However, the path to bringing senolytics from the laboratory to the clinic is fraught with challenges.
- One of the primary hurdles in drug development is securing adequate research funding. Funding sources often include government grants, private investments, and philanthropic contributions. These resources are essential for advancing clinical trials and covering the high costs of rigorous testing. Additionally, determining patient eligibility criteria stands as another significant challenge. Researchers must meticulously define which patient populations will most likely benefit from senolytic therapies, considering factors such as age, disease state, and genetic background.
- Ethical considerations also play a pivotal role in the development of analytics. The implications of extending the human lifespan, potentially altering life stages, and the equitable distribution of these therapies must be thoughtfully addressed. These discussions are essential for maintaining public trust and ensuring that treatments are developed in a manner that respects societal values and norms.
- Public awareness campaigns are essential to enhance understanding and support for this innovative field. These initiatives educate the broader community about the potential benefits and considerations of senolytic therapy, nurturing a sense of inclusion and anticipation. By engaging the public in meaningful dialogue, researchers and healthcare professionals can build a supportive environment that welcomes scientific advancements in combating the detrimental effects of aging.
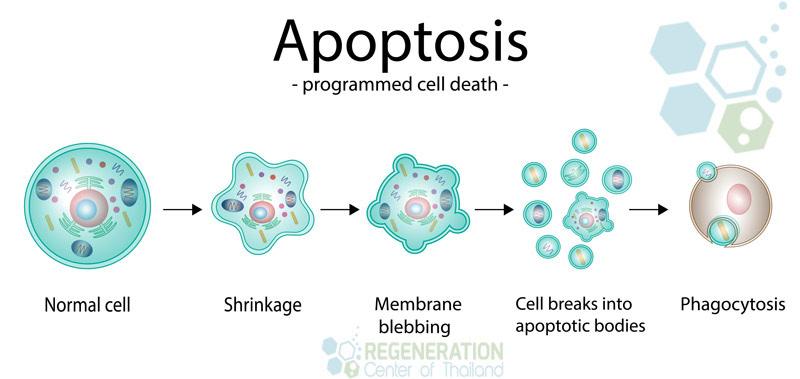
How Senolytic Therapy Works
Senolytic therapy operates by selectively targeting and eliminating senescent cells, which accumulate in tissues and contribute to various age-related diseases. This groundbreaking approach holds promise for enhancing health span and cultivates a sense of community among those seeking new avenues for age-related disease management. By understanding the mechanisms through which senolytics operate, we gain insight into a potentially revolutionary therapeutic strategy.
One pivotal aspect of senolytic therapy is the development of targeted drug delivery methods, CAR-T Cell Therapy and NK cell therapy. These methods enhance the efficacy of the treatment by ensuring that senolytic agents are delivered directly to the senescent cells. This specificity minimizes the impact on surrounding healthy cells, thereby reducing potential side effects—a critical factor in the acceptance and success of any new therapy.
Moreover, the therapy involves immune modulation to support the clearance of senescent cells. By fine-tuning the body’s immune response, senolytic therapy helps reinforce our natural ability to maintain optimal cellular health and homeostasis. This strategy is complemented by identifying specific genetic markers that indicate senescence, which aids in precisely targeting the problematic cells.
However, as with any therapeutic intervention, senolytic therapy comes with its side effects. These can vary widely among individuals but are an essential consideration for dosage optimization. The goal is to achieve the maximum therapeutic benefit with the minimum possible dosage, thereby reducing the risk of adverse effects while promoting a healthier, more dynamic community.
Through these sophisticated approaches, senolytic therapy represents a beacon of hope, uniting us in our quest for longevity and improved quality of life.
Clinical Trials and Current Treatment Options
Current clinical trials for senolytic therapies are exploring their efficacy and safety in treating age-related diseases. These trials are pivotal as they shed light on the benefits and potential risks associated with senolytic drugs. The methodologies used in these trials are meticulously designed to guarantee robust and reliable results. They often involve randomized control trials, the gold standard in clinical research.
Patient recruitment is a significant aspect, aiming to include diverse individuals to ensure the findings are widely applicable. Researchers focus on creating a sense of community among participants, emphasizing that their involvement is essential in advancing medical science and potentially improving health outcomes for many. This approach helps mitigate feelings of isolation and emphasizes the collective effort in tackling the challenges of age-related conditions.
Placebo effects are carefully considered and controlled in these studies, as they can skew the real impact of the tested treatment. This is achieved by having a control group that receives a placebo treatment, allowing researchers to measure actual drug effects more accurately. Safety monitoring is another critical component, with continuous oversight to promptly address any adverse effects that participants might experience during the trial.
Regulatory challenges also play a significant role, as new therapies must navigate complex approval processes before they can be widely used. Ensuring compliance with these regulations is essential for progressing senolytic therapies from the research phase to actual clinical use, offering hope and new solutions to those suffering from age-related diseases such as heart disease [4], osteoarthritis, diabetes, osteoporosis, congestive heart failure, strokes and cancer.
Future Directions in Senotherapy
As researchers continue to explore the potential of senolytic therapies, the future directions in senotherapy appear increasingly promising for addressing the complexities of age-related diseases. The integration of personalized medicine is at the forefront, tailoring treatments to individual genetic profiles and their unique disease pathways. This approach enhances the efficacy of senolytics and minimizes potential side effects, creating a sense of security and belonging among patients who receive care that feels specifically designed for them.[5]
Gene editing potential looms large in this scenario, offering possibilities to directly modify the genes responsible for cellular senescence. Techniques such as CRISPR could target and remove senescent cells more precisely, reducing the burden of damaged cells and improving overall tissue function. This precision speaks directly to the heart of personalized medicine and holds the promise of profoundly revolutionizing patient care.
Furthermore, understanding the immune system’s role in clearing senescent cells opens new avenues for immune modulation therapies. Enhancing the body’s natural ability to clear these cells can potentially delay the onset of senescence-associated disorders and many autoimmune diseases such as rheumatoid arthritis, ankylosing spondylitis, lupus, FMS, hashimotos and sjogren’s syndrome. This aligns directly with the our increasing interest in living longer and healthier lives.
The impact of dietary influences and lifestyle adjustments also continues to gain recognition. Nutritional protocols and exercise regimens that reduce senescent cell accumulation could enable individuals to take proactive steps towards managing their health, nurturing a sense of community and shared progress towards well-being. These holistic approaches complement high-tech treatments, ensuring that the future of senotherapy is both inclusive and all-encompassing.
How Does the Presence of Zombie Cells Affect the Body’s Response to Vaccines or Other Medical Treatments?
The presence of senescent, or ‘zombie’, cells can modulate immune function, potentially affecting vaccine efficacy. These cells often secrete pro-inflammatory factors, which could exacerbate the inflammation response to vaccines or treatments. Additionally, they might alter drug interactions or impair the body’s healing mechanisms. It is crucial for healthcare professionals to consider these implications to optimize patient care and treatment outcomes, ensuring that interventions are both effective and compassionate.
Implications for Longevity and Wellness
Building on the advancements in senotherapy, the implications for longevity and wellness are profound, promising a future where aging is not only delayed but accompanied by sustained health. As we explore the potential of senolytic therapies, it’s essential to recognize that individual lifestyle choices also play an important role in mediating the effects of aging.
Dietary impacts cannot be overstated; balanced, nutrient-rich diets can potentially reduce the prevalence of senescent cells, therefore amplifying the effectiveness of senolytic therapies. Foods high in antioxidants and anti-inflammatory properties may help stave off cellular senescence. Additionally, the benefits of exercise extend beyond mere physical fitness. Regular physical activity has been shown to enhance the body’s ability to clear senescent cells, improving longevity and the quality of life as we age.
Stress effects on cellular health are also significant. Chronic stress can accelerate the accumulation of senescent cells, thereby hastening the aging process. Managing stress through mindfulness practices, social support, and adequate relaxation is crucial for maintaining cellular health. Similarly, the correlation between sleep and senescence is becoming more apparent. Quality sleep helps regulate hormone levels that control cell repair and regeneration, suggesting that good sleep hygiene might be as critical as medical interventions in promoting health span.
Lastly, understanding hormone interactions offers another layer of insight into managing senescence. Hormones like insulin and cortisol directly impact cellular aging, and balancing these through lifestyle choices and potentially medical interventions might optimize senolytic therapy outcomes, making the path towards extended wellness inclusive and holistic.
Exploring zombie cells, or senescent cells, and their impact on aging and disease is fundamental for advancing regenerative medical science. Senolytic therapy emerges as a promising intervention to selectively eliminate these dysfunctional cells, potentially alleviating age-related decline and enhancing overall wellness. Our ongoing research is essential in validating these treatments in the clinical setting and in understanding their broader implications for longevity. The future of senotherapy holds significant promise for revolutionizing approaches to age-related conditions and wellness.
Published Clinical Citations
[1] ^Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99-118. doi: 10.1146/annurev-pathol-121808-102144. PMID: 20078217; PMCID: PMC4166495.
[2] ^Gazzillo A, Volponi C, Soldani C, Polidoro MA, Franceschini B, Lleo A, Bonavita E, Donadon M. Cellular Senescence in Liver Cancer: How Dying Cells Become “Zombie” Enemies. Biomedicines. 2023 Dec 21;12(1):26. doi: 10.3390/biomedicines12010026. PMID: 38275386; PMCID: PMC10813254.
[3] ^ Song P, An J, Zou MH. Immune Clearance of Senescent Cells to Combat Ageing and Chronic Diseases. Cells. 2020 Mar 10;9(3):671. doi: 10.3390/cells9030671. PMID: 32164335; PMCID: PMC7140645.
[4] ^ Saeedi Saravi SS, Feinberg MW. Can removal of zombie cells revitalize the aging cardiovascular system? Eur Heart J. 2024 Mar 14;45(11):867-869. doi: 10.1093/eurheartj/ehad849. PMID: 38190315.
[5] ^ Malayaperumal S, Marotta F, Kumar MM, Somasundaram I, Ayala A, Pinto MM, Banerjee A, Pathak S. The Emerging Role of Senotherapy in Cancer: A Comprehensive Review. Clin Pract. 2023 Jul 22;13(4):838-852. doi: 10.3390/clinpract13040076. PMID: 37489425; PMCID: PMC10366900.

