Chimeric antigen receptor-T cell treatment (CAR-T cell therapy) holds immense potential to revolutionize organ transplantation, particularly for patients who struggle to find a suitable match and are at high risk of rejection. This breakthrough therapy offers a beacon of hope, promising to significantly improve the outcomes and quality of life for these patients. Current research prioritizes using CAR-T cells obtained from the patient’s immune system to mitigate the risk of organ rejection following transplantation. Sensitized patients have elevated antibodies that elicit adverse immunological responses toward possible donor organs. These individuals frequently experience prolonged waiting periods for a kidney transplant.
This study represents a pioneering effort to assess the viability of employing immunotherapy to eliminate sensitizing B cells in patients awaiting kidney transplantation. It is the first step towards utilizing CAR-T cells in transplantation, with the ultimate goal of increasing the availability of donor organs for transplantation and alleviating the waiting time for patients in need of a new kidney.
What is CAR-T Cell Therapy?
CAR-T cell therapy, also known as Chimeric Antigen Receptor T-cell treatment, is an immunotherapy technique that harnesses a patient’s modified T cells to combat cancer. CAR-T cell therapies involve genetically modified immune cells in a lab to target and destroy the patients cancer cells.
- T-cell harvesting: T lymphocytes, a subset of leukocytes, are obtained from the patient’s bloodstream. Genetic modification manipulates T cells to develop chimeric antigen receptors (CARs) on their surface in a laboratory. These chimeric antigen receptors (CARs) enable T lymphocytes to identify and bind to particular proteins (antigens) found in cancerous cells.
- T-cell proliferation: The genetically engineered T cells are cultivated in a controlled environment until their numbers reach millions. Cancer screening required
- Administration: The CAR-T cells are subsequently administered into the patient’s circulatory system.
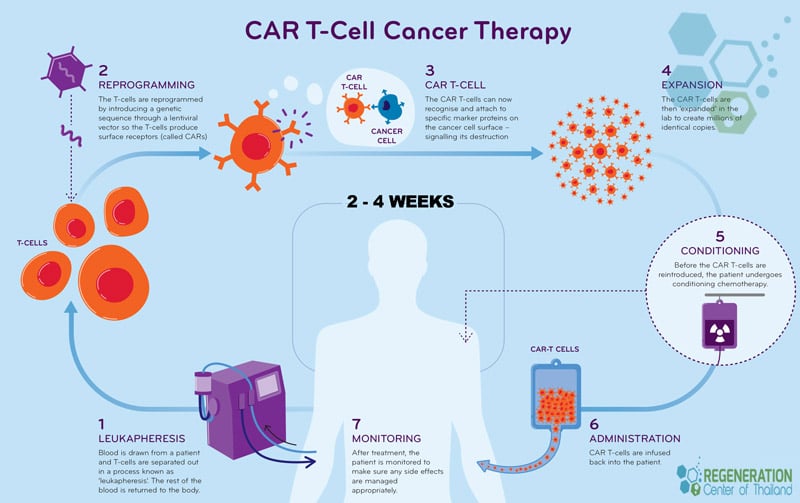
The genetically modified CAR-T cells undergo rapid replication within the patient’s body. These cells can identify and eliminate cancer cells that display a particular antigen on their outer surfaces. CAR-T cell therapy has already shown promising results in treating specific blood malignancies, such as acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL). These encouraging outcomes provide a strong foundation for further research, instilling confidence in the potential of CAR-T cell therapy in other medical applications, including organ transplantation, stem cell therapy for kidney disease, stem cell treatment for PKD, metabolic disorders and stem cell therapy for diabetic nephropathy.
Investigation of the efficacy of CAR-T cell treatment and the process of transplantation.
Scientists have created a novel form of CAR-T cell treatment that specifically targets a protein called B cell activating factor receptor (BAFF-R) found in B cells. B cells are crucial in controlling the immune system and producing antibodies. These altered CAR-T cells can precisely identify and eliminate B cells responsible for sensitization.
How does the treatment work?
Scientists first produced chimeric antigen receptor T cells (CAR-T cells) from 15 patients who were identified as being sensitized to organ transplantation. Eight individuals had undergone renal transplants, while one had received a pancreatic transplant. By extracting T cells from approximately 100 milliliters of the patient’s blood. These CAR-T cells can be modified to recognize and deactivate B cells that possess a particular antigen, thereby efficiently focusing on and eradicating the cells that cause sensitization.
Subsequently, the researchers compared the activity of the genetically engineered CAR-T cells and T cells that had been subjected to treatment with B cells obtained from healthy donors. The researchers observed a decrease in B cells in the group of sensitized patients who received treatment with patient-derived CAR-T cells compared to the control group that did not receive CAR-T cells. This study provides compelling evidence that CAR-T cells produced from sensitized patients effectively eliminate B cells. This supports the potential use of CAR-T cell therapy as a desensitization technique for patients seeking kidney transplants. These findings underscore the promising role of CAR-T cell therapy in the field of organ transplantation.
The application of CAR-T cell therapy for desensitizing kidney transplant patients is in the experimental phase. However, it represents one of the initial investigations into the utilization of this immunotherapy for metabolism cancers or for conditions other than cancer treatments. The Regeneration Center provides CAR-T Cell treatments and NK cells for treating various forms of cancer, including kidney cancer, lung cancer, pancreatic cancer, prostate cancer, liver cancer, colorectal cancer, multiple myeloma, and mesothelioma cancer.
What is the difference betweeen CAR T cells and. NK Cells?
The distinction between CAR T cells and NK cells lies in their functional characteristics and mechanisms of action. CAR-T cells and NK cells are components of the immune system. However, they possess essential dissimilarities:
- Origin: CAR-T cells are T cells that have undergone genetic modification in a laboratory setting to produce chimeric antigen receptors (CARs).
- NK cells, or Natural Killer cells, are a subset of leukocytes integral to the innate immune response and do not necessitate genetic alteration.
- Specificity: CAR-T cells are designed to selectively recognize and bind to particular antigens found on cancer cells, rendering them exceptionally precise in their therapeutic effect.
- Natural killer (NK) cells can identify and eliminate cells that exhibit irregularities, such as cancerous cells or cells infected by viruses, without the need for prior sensitization.
- Persistence: Following their introduction through infusion, CAR-T cells can remain in the body for extended periods, ranging from months to even years. This prolonged presence offers a durable defense against the reappearance of cancer.
- Natural killer (NK) cells exhibit a reduced lifespan and do not confer durable immunity.
- Adverse consequences: CAR-T cell therapy can induce significant adverse effects, including cytokine release syndrome (CRS) and neurotoxicity, due to the robust immunological response elicited by the transformed T cells.
- Natural killer (NK) cell therapy often has a reduced likelihood of significant adverse effects compared to chimeric antigen receptor T-cell (CAR-T) therapy.
- Medical applications: CAR-T cell therapy has effectively treated several hematologic malignancies, including acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL).
The Regeneration Center currently offers NK cell therapy as a potential treatment for several forms of cancer, including solid tumors. Several patients who had treatment have demonstrated encouraging results. CAR-T and NK cells are not just areas of research, but significant focal points in the dynamic field of cancer immunotherapy. Understanding their distinct benefits and challenges is crucial for staying informed and engaged in the latest advancements in medical science.