Vascular enlargement, or angiogenesis, occurs when new blood vessels form inside the body’s preexisting vascular system. It begins in utero until old age, in health and sickness. A blood capillary, generated by the process of angiogenesis, is never more than a few hundred micrometres away from any metabolically active tissue in the body. All tissues rely on capillaries to facilitate the diffusional exchange of nutrients and metabolites. Angiogenesis and, by extension, veins respond proportionally to variations in metabolic activity. The involvement of oxygen in this control system is crucial. Patients with COPD and IPF understand that survival of vascular networks and structural modifications of vessel walls depend on hemodynamic variables.[1]
Brief Overview of Angiogenesis
Over the past 40 years, researchers have paid close attention to the possibility that angiogenesis regulation might have therapeutic benefits. In ischemic heart disease, peripheral artery disease, and wound healing, stimulation of angiogenesis might be beneficial. Angiogenesis is a critical process in developing new blood vessels, and preventing or slowing their growth can benefit various disorders. Capillaries expand and contract in response to the needs of healthy tissues. Skeletal muscle and the heart are two organs that benefit significantly from angiogenesis stimulation by physical activity. Capillary regression is a result of inactivity. Adipose tissue capillaries expand with weight increase and contract with weight decrease. The process of angiogenesis involves multiple elements in the body is ongoing and constant.[2]
Angiogenesis Subtypes
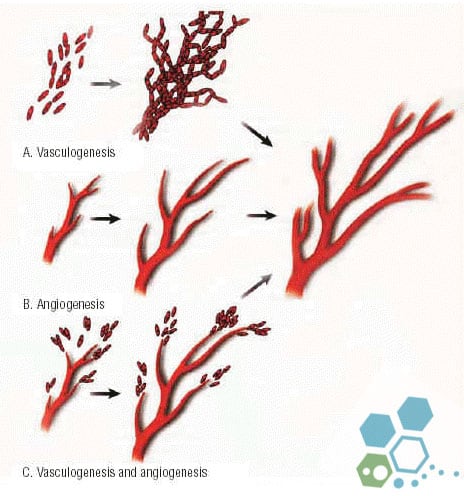
Angiogenesis can occur both in utero and later in life, and it can take two forms: sprouting and intussusceptive. Although the process of sprouting angiogenesis was first identified over 210 years ago, the process of intussusceptive angiogenesis was first identified roughly 30 years ago by Burri. Endothelial cell sprouts, as suggested by the name, are the defining feature of sprouting angiogenesis, and they typically develop in the direction of an angiogenic stimulus, such as VEGF-A. Therefore, areas of tissue lacking blood vessels might have grown thanks to sprouting angiogenesis. However, in intussusceptive angiogenesis, pieces of interstitial tissues enter preexisting arteries to generate expanding transvascular tissue pillars; this is how new blood vessels are formed. It is widely believed that both angiogenesis forms occur in all tissues and organs.[3]
Role of Angiogenesis in Stem Cell Therapies
Diseases like heart failure are often characterized by either insufficient vascularization or aberrant vasculature, suggesting that angiogenesis may be a target for a fight.
 Treating these disorders may benefit from administering drugs that can prevent or stimulate new blood vessels’ development. Blood vessels in places where there shouldn’t be any can change the mechanical characteristics of tissue, making it more vulnerable to breakdown. Repair or other metabolically active tissue may be hampered if it lacks enough blood supply resulting in common symptoms such as brain fog. Ischemic heart disease and heart attacks are other conditions that can be treated better by increasing blood flow to the area since this will supply fresh nutrients to the damaged tissue and promote healing. Ageing-related macular degeneration is one illness that may originate from abnormal vasculature growth that disrupts normal physiology.
Treating these disorders may benefit from administering drugs that can prevent or stimulate new blood vessels’ development. Blood vessels in places where there shouldn’t be any can change the mechanical characteristics of tissue, making it more vulnerable to breakdown. Repair or other metabolically active tissue may be hampered if it lacks enough blood supply resulting in common symptoms such as brain fog. Ischemic heart disease and heart attacks are other conditions that can be treated better by increasing blood flow to the area since this will supply fresh nutrients to the damaged tissue and promote healing. Ageing-related macular degeneration is one illness that may originate from abnormal vasculature growth that disrupts normal physiology.
Antiangiogenic treatments, where angiogenic research first started, and pro-angiogenic therapies make up the two primary areas of clinical application of the principle of angiogenesis today. Pro-angiogenic medicines are being investigated as alternatives for treating cardiovascular disorders causing hear failure, one of the leading cause of mortality in the world. In contrast, antiangiogenic therapies combat cancer and malignancies, which require excess oxygen and nutrients to flourish. A clinical experiment employing fibroblast growth factor 1 (FGF-1) for treating IPF, coronary artery disease and COPD were one of the earliest uses of pro-angiogenic therapies.
[4]
Gene therapy, which amplifies or inhibits genes of interest; protein replacement therapy, which primarily manipulates angiogenic growth factors like FGF-1 or vascular endothelial growth factor, VEGF; and cell-based therapies, which involve the implantation of specific cell types, are the three main categories of pro-angiogenic methods based on their mechanism of action.
Concerning gene therapy, there are still significant issues that need to be addressed. The genetic complexity of angiogenesis and the viral vectors used to implant therapeutic genes present substantial challenges. Other problems include ensuring the efficient integration of the isolated genes into the genome of target cells and minimizing the risk of an unwanted immune response, toxicity, immunogenicity, inflammatory responses, and oncogenesis. Injecting a single gene may not be particularly helpful in diseases such as strokes, spinal cord injury, diabetes, and Alzheimer’s since these conditions are likely caused by the cumulative impact of mutations in numerous genes.
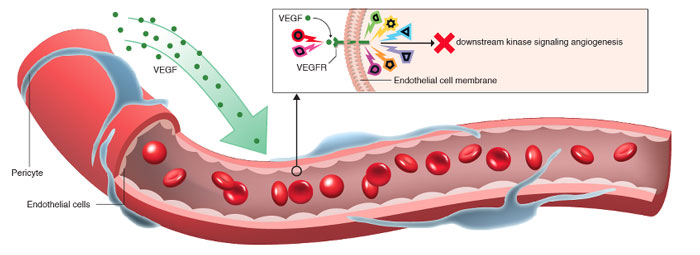
In contrast, pro-angiogenic protein therapy uses well-defined, precisely structured proteins, the appropriate dosages of which have been determined for specific disease states and the biological effects of which are well-established. However, protein treatment has challenges associated with its distribution method. Proteins may not be as effective when administered orally, intravenously, intraarterially, or intramuscularly because they may be digested or eliminated before reaching the intended area. Numerous unanswered concerns about the optimal cell kinds and doses to utilize in cell-based pro-angiogenic treatments using allogeneic cells are still in the early phases of research.
Angiogenesis in tumours
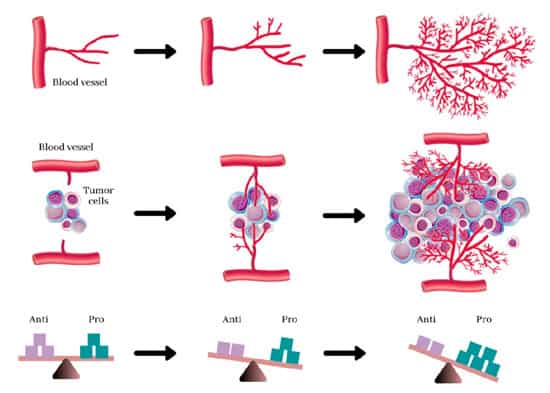
A tumour’s growth is capped without angiogenesis. The inability to divide is typically what sets cancer cells apart. Rapidly replicating cancer cells that have accumulated mutations over time make up a malignant tumor. Tumors can only develop beyond a specific size if they get a constant supply of oxygen and nutrients from a specially designed blood supply.
Growth factors (such as VEGF) and other substances secreted by tumours promote the growth of new blood vessels. Some researchers believe that tumour growth hormones like bFGF and VEGF cause capillary development in the tumour, which supplies cancer with nutrition. Tumor blood vessels, on the other hand, are enlarged and asymmetrical. Some doctors think angiogenesis is just a trash disposal system to eliminate the byproducts of fast cell division in cancer. Regardless, angiogenesis is a crucial stage in the progression of a tumor from a small benign cluster of cells, roughly the size of the metal ball at the end of a ball-point pen, to a massive tumor. Angiogenesis is also necessary for cancer to metastasize or spread to other body parts. Isolated cancer cells can travel via the bloodstream from an existing solid tumor to a new location, where they can implant and start a new tumor. We now know that the blood vessels in a particular solid tumor may be mosaic vessels made up of endothelial cells and tumor cells. This mosaicism permits significant shedding of tumor cells into the vasculature, which may contribute to the emergence of circulating tumor cells in the peripheral blood of patients with malignancies. Furthermore, such metastases will need access to good nutrition, oxygen, and a means of getting rid of waste in order to grow and spread.
It has long been believed that endothelial cells are more genetically stable than cancer cells. Compared to chemotherapy, which is focused on cancer cells, antiangiogenic therapy targeting endothelial cells has an advantage due to their genetic stability, preventing them from rapidly mutating and acquiring drug resistance. This has led many to believe that endothelial cells might be an excellent therapeutic target.
Treatment of cardiovascular disease via angiogenesis
With its potential as a therapeutic target, angiogenesis offers hope for treating cardiovascular disease. The body’s natural reaction to a decrease in blood flow to essential organs is neoangiogenesis, the formation of new collateral vessels to counteract the ischemic shock. Numerous protein, gene, and cell-based therapeutics have been studied extensively in models of liver failure and peripheral artery disease before they were brought into clinical trials. Early animal trials showed promising results, leading to solid hopes that this novel medicinal method will soon be able to help millions of people in the West who suffer from similar conditions. But despite a decade of clinical testing, gene and protein-based treatments intended to induce angiogenesis in under-perfused tissues and organs have failed dismally. Despite the incorporation of these preclinical readouts, which showed significant promise for translating angiogenesis treatment for limited groups of patients with the primary goal for developing a standardized protocol using angiogenic therapies.
These setbacks imply that either these are not the suitable molecular targets to induce neovascularization, require careful formulation and administration to be effective, or that how they are presented within the larger context of the cellular microenvironment may be crucial. The concentration, geographical, and temporal characteristics of these protein presentations and their potential concomitant or sequential presentation with other relevant components may need to imitate natural signalling processes.
What is neuroangiogenesis?
The term “neuroangiogenesis” describes new nerves and blood vessels developing in tandem. This coordinated expansion is enabled by the everyday use of guidance signals and cell-surface receptors across the neurological and vascular systems. The process was referred to as neurovascular patterning before 2012 when the word neuroangiogenesis was first used to describe it. Neurogenesis and angiogenesis work hand in hand during early life and development. Some believe it has a role in developing endometriosis, malignancies,brain injuries, strokes, acute spinal cord injuries and perhaps neurodegenerative diseases like, Parkinson’s, ALS, MND & Alzheimer’s disease.
Chemotherapeutic medicines and Metronomic therapies are being utilized to treat various diseases, including kidney cancer, pancreatic adenocarcinoma, liver cancer, lung carcinoma and prostate cancer. There were substantial side effects and bystander effects from several drugs, yet they were only partially effective because of problems with administration, penetration, and selectivity for tumor cells. The drug resistance of tumour cells primarily limits the effectiveness of these medicines. Evidence from animal and human investigations of angiogenesis-based treatment suggests it may have future applications in healthcare. Since it does not directly target cancer cells but rather the formation of blood vessels, antiangiogenic treatment is an innovative method for eliminating tumor cells. Antiangiogenic drugs have not been shown to cause hair loss, gastrointestinal side effects resulting in IBD, MCTD, or inhibition of bone marrow. Clinical studies should consider that the strategy to restrict blood vessel formation may take many months to a year and may require medicines to be administered at lower dosages and for more extended continuous periods than traditional cytotoxic agents.[5] Resistance to angiogenic inhibitors has not been a significant concern thus far. Furthermore, combining antiangiogenic treatment with standard care may yield better outcomes than either approach alone. Therapy based on angiogenesis shows promise as a unique, selective, safe, and rational therapeutic option for the future of medicine.
Published Clinical Citations
[1] ^Fang S, Salven P. Stem cells in tumor angiogenesis. J Mol Cell Cardiol. 2011 Feb;50(2):290-5. doi: 10.1016/j.yjmcc.2010.10.024. Epub 2010 Nov 1. PMID: 21047516.
[2] ^ Melero-Martin JM, Dudley AC. Concise review: Vascular stem cells and tumor angiogenesis. Stem Cells. 2011 Feb;29(2):163-8. doi: 10.1002/stem.583. PMID: 21732475; PMCID: PMC3083523.
[3] ^ Tang YP, Liu Y, Fan YJ, Zhao YY, Feng JQ, Liu Y. To develop a novel animal model of myocardial infarction: A research imperative. Animal Model Exp Med. 2018 Apr 19;1(1):36-39. doi: 10.1002/ame2.12010. PMID: 30891545; PMCID: PMC6357429.
[4] ^De Falco S. Antiangiogenesis therapy: an update after the first decade. Korean J Intern Med. 2014 Jan;29(1):1-11. doi: 10.3904/kjim.2014.29.1.1. Epub 2014 Jan 2. PMID: 24574826; PMCID: PMC3932378.
[5] ^Nensat C, Songjang W, Tohtong R, Suthiphongchai T, Phimsen S, Rattanasinganchan P, Metheenukul P, Kumphune S, Jiraviriyakul A. Porcine placenta extract improves high-glucose-induced angiogenesis impairment. BMC Complement Med Ther. 2021 Feb 18;21(1):66. doi: 10.1186/s12906-021-03243-z. PMID: 33602182; PMCID: PMC7893890.

