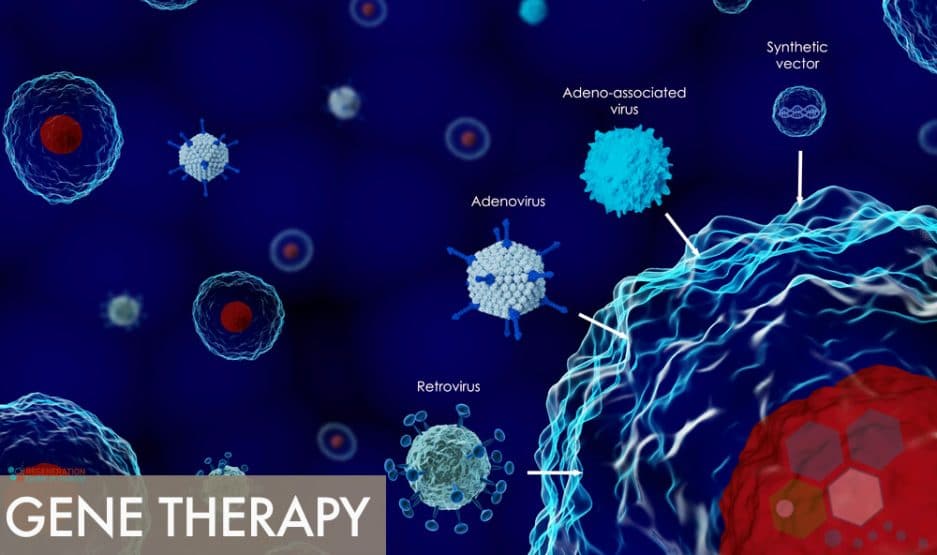
Most traditional treatments today are designed using a one-size-fits-all to cover the “average” patient. But advances in biotechnology and Artificial intelligence (AI) medical researchers are now equipped with vast amounts of personalized datasets at their disposal, and can analyze unique situations based on our genetic profiles, family medical histories and other relevant medical conditions to better understand what types of therapies work best for each segments of the human population.
This laser targeted approach to healthcare is a huge deal, and billions are being invested to fund biologists, geneticists, computer scientists and experts in AI (artificial intelligence) to more accurately predict how specific genetic mutations around the world will respond to such personalized treatments. We are slowly building a map of the human genetic variations giving regenerative medical physicians better tools to craft medical therapies and achieve better response rates with less risk. The regeneration center offers functional medical programs using Genomics to customize nutrition and wellness recommendations based on each patients unique DNA profiles. Many new gene therapies currently being tested are not looking to correct the genetic defects direct but instead are looking to introduce a new copy of altered genes with the hopes of fixing the underlying issues.[1]
One such example is the recent Cure for Beta-Thalassemia in Thailand using modified globin genes. β-hemoglobinopathies such as β-thalassemia and sickle cell disease, are some of the most prevalent monogenic disorders around the world and quite frequent in Thailand where nearly 3,500 babies are born each year with beta-thalassemia disease. This disorder requires monthly blood transfusions for the entire life of the patient. Even with previous treatment options, complications are frequent, and patient life expectancy is quite short. Through the cooperation of the Institute of Personalized Genomics and Gene Therapy at Mahidol University in Thailand and Harvard Medical School, the team recently announced a groundbreaking method for curing thalassemia by autologous gene therapy.[2] Although the treatment is currently still in clinical trials, the results of the first group of patients are published in The New England Journal of Medicine.
Other Areas Experimenting with Gene Therapies:
Autoimmune Diseases – Understanding the molecular basis of autoimmune conditions have helped create new modified gene therapies to treat affected patients who previously did not have  any options. The main benefits of gene therapy in the treatments of autoimmune conditions is to help restore ‘immune homeostasis’ by hindering the inflammatory effects of T cells. The modified T cell therapy helps to better regulate proinflammatory cytokines and infiltration of lymphocytes to the affected areas in the body.[3] Over 12 clinical trials are currently taking place around the world looking to treat autoimmune diseases like rheumatoid arthritis, Juvenile idiopathic arthritis, insulin-dependent type 1 diabetes, multiple sclerosis, systemic lupus, autoimmune encephalomyelitis [4] and other rare diseases such as inclusion body myositis a condition that musician Peter Frampton was recently diagnosed with.
any options. The main benefits of gene therapy in the treatments of autoimmune conditions is to help restore ‘immune homeostasis’ by hindering the inflammatory effects of T cells. The modified T cell therapy helps to better regulate proinflammatory cytokines and infiltration of lymphocytes to the affected areas in the body.[3] Over 12 clinical trials are currently taking place around the world looking to treat autoimmune diseases like rheumatoid arthritis, Juvenile idiopathic arthritis, insulin-dependent type 1 diabetes, multiple sclerosis, systemic lupus, autoimmune encephalomyelitis [4] and other rare diseases such as inclusion body myositis a condition that musician Peter Frampton was recently diagnosed with.
Anti-aging – Ageing is a disease. Gene therapy could be the cure. Current Anti-aging treatments offer only slight benefits for men and women dealing with the aging process. Research so far has shown that lifestyle modifications have minimal impact in treating diseases of age and it appears that biotechnology and gene therapies might be the best solution. In the Aging or sustained cell division process, telomeres act as natural buffers for wear and tear but eventually get too short and are unable to protect the chromosomes. When they get too short, it causes the cells to malfunction in programmed cell death which leads to what we call “aging.” Some researchers are conducting clinical trials for breakthrough telomerase gene therapy that has shown to be powerful enough to slow down age-related pathologies which help extend longevity. Shorter telomere lengths have been shown to have a direct relationship with increased risk in cardiovascular diseases and many types of cancers including prostate cancer.
Hereditary Brain Disorders – Biotechnology companies and researchers have begun offering new treatments and customized gene therapies using Crispr for many rare genetic disorders that can be adapted to treat other inherited disorders such as Huntington’s disease and leukodystrophies that affect the white matter in the brain. The novel new treatment involves naturally boosting then collecting specific stem cells from patients peripheral blood them editing the DNA in the stem cells with CRISPR-Cas 9 technology then delivering the modified cells back into the patient for a real cure.[5]
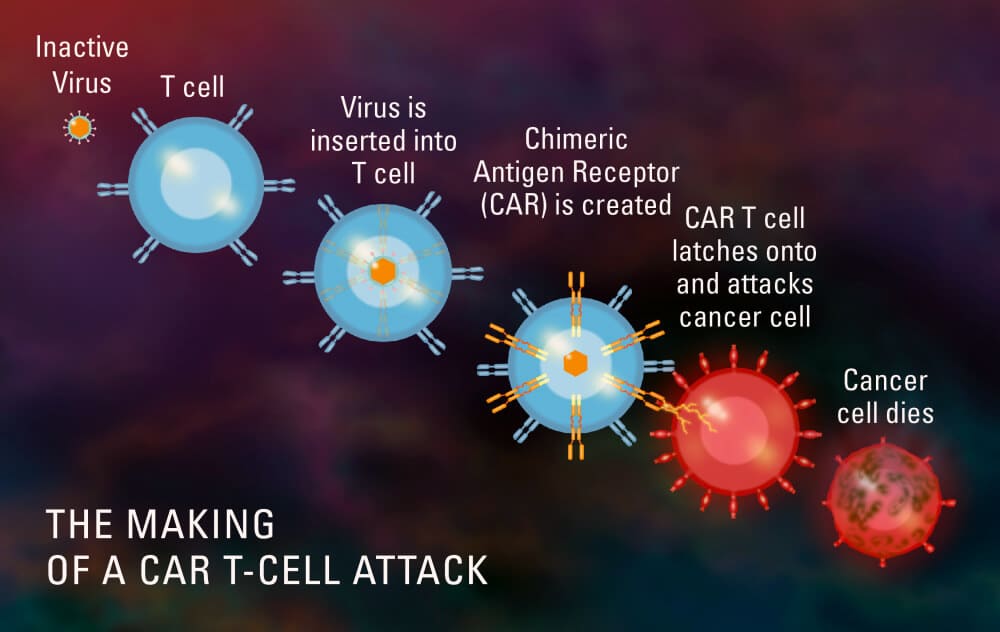
Cancer – Cancer gene therapies and T-Cell treatments were first approved for use by the US FDA as recently as 2017 for treating pediatric and adult patients acute lymphoblastic leukemia (ALL). Cancer immunotherapies are a new frontier in medical innovation & biotechnology. The treatments use the patient’s own cells then reprogram them to attacks the cancer cells in the patient’s body. Recent clinical trials have been very successful with nearly 85 percent remission rates using a combination treatment with NK cells for many types of cancers including lung, prostate and pancreatic tumours. Other types of diseases that are being targeted included squamous cell cancer of the neck, ovarian cancer, breast cancer, liver cancer, bladder cancer, and brain cancer.[6]
Other new gene therapies currently being tested are cancer vaccines. Vaccines require the collection of tumor cells directly from the patient and editing them with a specific genetic encoding that causes them to be much more visible to the immune system defense mechanisms. The modified cells are edited then reintroduced back into the patient along with immune-stimulating growth factors. After infusion, the patient’s immune system immediately recognizes the modified cells and launches a full attack on the freshly-infused cancer cells & also any other similar cells in the body that were previously ignored by the immune system.
Vision Loss and Blindness – Until recently, the genetic cause of blindness have been untreatable. The idea of gene therapy for vision-related diseases seeks to address them on the DNA level by using injections of modified retinal cells using a virus as the carrier. The viral transportation method allows the missing gene to correct the root cause of the disease resulting in long term beneficial effects on optic nerve cells to reverse blindness. European regulators recently approved new gene therapy to restore vision for patients with a specific genetic mutation of RPE65 that causes the retinal cells to die over time, resulting in loss of vision slowly. Currently, in the Phase III clinical trial, the Luxturna gene therapy is reported to use a single infusion therapy to improve vision in as little as one month after treatment.[7]
Lung Diseases and Hereditary Asthma – New pulmonary gene therapies are looking to treat idiopathic lung diseases that were caused by both genetic and environmental conditions.[8] One such treatment uses modified cells to ‘turn-off’ the bodies immune system response which results in allergic reactions to asthma or even food allergies. The current challenge or treating conditions due to allergies or asthma is that T-cells quickly develop a ‘memory’ making them resistant to traditional treatments methods. By altering the Immune T-Cells, doctors will be able to clean the memory of these cells allowing the immune system to tolerate the proteins that cause the undesired immune reactions. Other Gene therapies in clinical trials for Lung Diseases include:
- Cystic Fibrosis [9]
- Lung cancer [10]
- Acute lung injuries such as COPD, Emphysema and IPF [11]
Gene therapy for lungs uses nucleic acids such as DNA or RNA as a delivery vehicle into the body to facilitate the desired expressions of proteins. Gene therapies are implemented using gene addition (gene replacement), gene repair or gene reprogramming. Currently, the most common treatments use Gene addition as the primary method of treating inherited genetic mutations.
Spinal Cord Injuries – Stitching Together Damaged Spinal Cords? Experimental new gene therapy is being tested in paralyzed mice helping them walk and grab again. When the spinal cord gets damaged, patients lose a little or all ability to control parts of the body and limbs. For most spinal cord injury patients’ and neurological conditions, the formation of scar tissue blocks the neural and nerve cells from communicating with the brain, each other and with any muscles that they control. A new spinal gene therapy looks to break down any scar tissue thus enabling neurogenesis at the site of allowing patients with control over their previously paralyzed limbs. For most patients treated for spinal cord injuries, recovering the use of hands is usually the top priority. If successful, the new gene therapy would allow such improvements in just a few weeks are enabling spinal cord injury patients to wash once again or dress independently and eat without assistance resulting in a significant improvement in the overall quality of life.[12]
Parkinson’s Disease – After a few months, Patients diagnosed with Parkinson’s are usually very disappointed with the results of L-Dopa (levodopa). Over time the medications become much
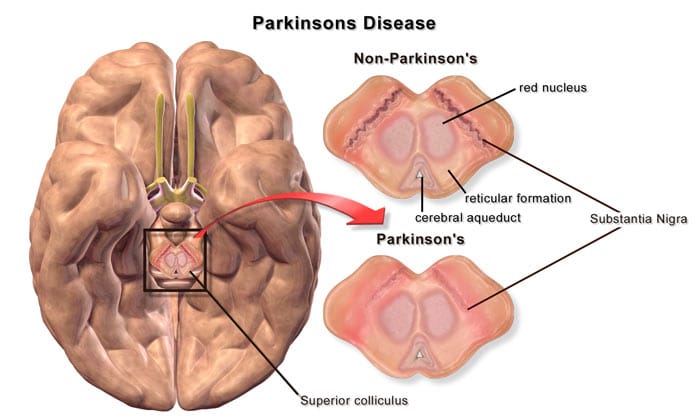
less effective or require incredibly high doses resulting in a state of nearly frozen paralysis. The reason these medications fail over time is that the human brain also starts losing the AADC enzyme (aromatic L-amino acid decarboxylase) that is critical in the conversion or L-Dopa into dopamine. Unlike conventional studies, gene therapy for Parkinson’s uses a modified virus called adeno-associated viral vector (AAV) to carry the gene needed to produce AADC enzyme in the brain. The gene-modified viral particles are then the replacement cells are derived back into the brain and nervous system using guided radiograph technology. MRI scans taken after treatment show new links formations between the cortical premotor, basal ganglia and motor regions.[13]
Multiple Sclerosis – Multiple sclerosis starts as an inflammatory disease which if left untreated turns into neurodegenerative disease. Gene therapy for MS and Ataxia allows modified allogeneic cells that help induce larger populations of regulatory cells such as markers CD4, CD25, and FOXP3 that are responsible for suppressing the inflammation. The results of this new therapy for MS are promising and have already shown the ability for long-term stoppage and reversal of MS.
Tracking Changes in Cancers on a Global Level
Another advancement in epigenomics, genetics, and biotechnology is the study of cancers and how they mutate after traditional treatments. Understanding who or what turns our

genes on and off can help us understand how tumors mutate. Such knowledge is crucial for doctors so they can develop new immunotherapy protocols that are resistant to mutations allowing us to target tumor cells like ordinary and manageable chronic conditions.[14]
Every cell in the human body carries the same genetic coding however cells in the brain, heart, skin and bone function very differently based on the chemical signaling that can either activate or silence genetic expressions. This nature vs nurture response is unique and challenging to predict patient to patient. Disruptions to this signaling mechanism are known to cause most major diseases including Diabetes, lung cancers or neurological conditions such as Alzheimer’s and MND. Learn more in the stem cell glossary.
Understanding these unique circumstances on the molecular level gives doctors the power to find the optimal options for each condition the patient might be facing and how they might respond to particular treatment even before the procedure begins.
The Beginning of Precision Medicine
The challenge for gene and exosome therapies is finding ways to administer a very complex, life-saving treatment in an affordable manner and especially in areas of the world where healthcare is limited, to begin with. Although cord blood and marrow stem cell transplants exist throughout the world, the ability to sequence genes in an efficient way is not widely available.
There is a sound humanitarian argument in favor of funding and building more gene therapy trials around the globe. Beta thalassemia and sickle cell disease are very expensive to treat over the long term of a patient’s life and modified gene therapies offer doctors to cure with a single-dose prescription of modified cells. These genetic diseases result in life long disability and often result in early death. Science and medical researchers have once again found solutions to previously impossible conditions and will continue to do so if we make healthcare a priority.
Published Clinical Citations
[1] ^ Cappa, Ryan, Liana Theroux, and J Nicholas Brenton. 2017. Pediatric Multiple Sclerosis: Genes, Environment, and a Comprehensive Therapeutic Approach. Pediatric neurology (July 13). doi:S0887-8994(17)30297-7. https://www.ncbi.nlm.nih.gov/pubmed/28843454
[2] ^ Cha, Charles W, and Scott D Boden. 2003. Gene therapy applications for spine fusion. Spine, no. 15 Suppl ( 1). https://www.ncbi.nlm.nih.gov/pubmed/12897478
[3] ^ Chen, Y, V K Kuchroo, J Inobe, D A Hafler, and H L Weiner. 1994. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science (New York, N.Y.), no. 5176 ( 26). https://www.ncbi.nlm.nih.gov/pubmed/7520605
[4] ^ Choong, Chi-Jing, Kousuke Baba, and Hideki Mochizuki. 2015. Gene therapy for neurological disorders. Expert opinion on biological therapy, no. 2 (December 5). doi:10.1517/14712598.2016.1114096. https://www.ncbi.nlm.nih.gov/pubmed/ 26642082
[5] ^ Choong, Chi-Jing, and Hideki Mochizuki. 2016. Gene therapy targeting mitochondrial pathway in Parkinson’s disease. Journal of neural transmission (Vienna, Austria : 1996), no. 2 (September 16). doi:10.1007/s00702-016-1616-4. https://www.ncbi.nlm.nih.gov/pubmed/27638713
[6] ^ Cmielewski, Patricia, Nigel Farrow, Sharnna Devereux, David Parsons, and Martin Donnelley. 2017. Gene therapy for Cystic Fibrosis: Improved delivery techniques and conditioning with lysophosphatidylcholine enhance lentiviral gene transfer in mouse lung airways. Experimental lung research, no. 9-10 (December 13). doi:10.1080/01902148.2017.1395931. https://www.ncbi.nlm.nih.gov/pubmed/29236544.
[7] ^ Dalkara, Deniz, Olivier Goureau, Katia Marazova, and José-Alain Sahel. 2016. Let There Be Light: Gene and Cell Therapy for Blindness. Human gene therapy, no. 2. doi:10.1089/hum.2015.147. https://www.ncbi.nlm.nih.gov/pubmed/ 26751519.
[8] ^ Husain, S R, J Han, P Au, K Shannon, and R K Puri. 2015. Gene therapy for cancer: regulatory considerations for approval. Cancer gene therapy, no. 12 (November 20). doi:10.1038/cgt.2015.58. https://www.ncbi.nlm.nih.gov/pubmed/26584531.
[9] ^ Kim, Namho, Gregg A Duncan, Justin Hanes, and Jung Soo Suk. 2016. Barriers to inhaled gene therapy of obstructive lung diseases: A review. Journal of controlled release : official journal of the Controlled Release Society (May 16). doi:S0168-3659(16)30310-8. https://www.ncbi.nlm.nih.gov/pubmed/ 27196742.
[10] ^ Biasco, Luca. 2017. Integration Site Analysis in Gene Therapy Patients: Expectations and Reality. Human gene therapy, no. 12 (December 0). doi:10.1089/hum.2017.183. https://www.ncbi.nlm.nih.gov/pubmed/29160103
[11] ^ Lara-Guerra, Humberto, and Jack A Roth. 2016. Gene Therapy for Lung Cancer. Critical reviews in oncogenesis, no. 1-2. doi:10.1615/CritRevOncog.2016016084. https://www.ncbi.nlm.nih.gov/pubmed/27481008.
[12] ^ Macer, D R, S Akiyama, A T Alora, Y Asada, J Azariah, H Azariah, M V Boost, P Chatwachirawong, Y Kato, and V Kaushik. 1995. International perceptions and approval of gene therapy. Human gene therapy, no. 6. https://www.ncbi.nlm.nih.gov/pubmed/7548279.
[13] ^ Stabler, Collin T, and Edward E Morrisey. 2016. Developmental pathways in lung regeneration. Cell and tissue research, no. 3 (December 13). doi:10.1007/s00441-016-2537-0. https://www.ncbi.nlm.nih.gov/pubmed/27957616
[14] ^ Wirth, Thomas, Nigel Parker, and Seppo Ylä-Herttuala. 2013. History of gene therapy. Gene, no. 2 (April 23). doi:10.1016/j.gene.2013.03.137. https://www.ncbi.nlm.nih.gov/pubmed/23618815