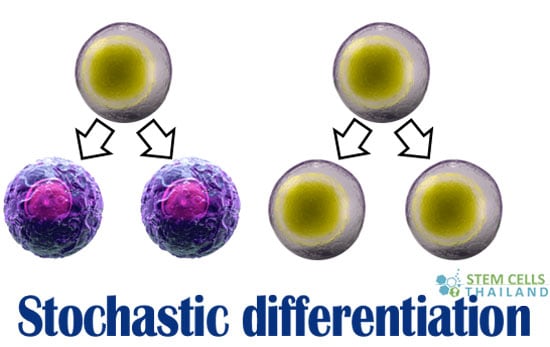
Stochastic differentiation refers to the process in which a stem cell undergoes division, giving rise to two daughter cells that are differentiated.[1]
On the other hand, another stem cell goes through the process of differentiation or mitosis to generate two stem cells that are similar to its original cell state, thereby maintaining stem cell number.[2] This process of differentiation is necessary for stem cells to maintain their number (stem cell reserve) in the body.[3]
Stochastic differentiation refers to the process of cellular differentiation that is influenced by random events, rather than purely deterministic, pre-programmed pathways. In the context of stem cell biology and development, the differentiation of cells can be influenced by a combination of intrinsic and extrinsic factors. While certain differentiation pathways are deterministic and driven by specific molecular cues, stochastic events can introduce variability into these processes.
What is stochastic differentiation in stem cells?
When one father stem cell divides into 2 differentiated daughter cells. a second father cell divides into two stem cells to maintain the population.
- Intrinsic Noise: Within cells, random fluctuations in the levels or activity of molecules (e.g., mRNA, proteins) can arise due to the inherent stochasticity of biochemical reactions. Such fluctuations are termed “intrinsic noise” and can influence cellular decisions, especially when the number of molecules involved is low.
- Extrinsic Noise: This type of noise arises from variations in the cellular environment. For instance, neighboring cells might release variable amounts of signaling molecules, or there might be fluctuations in nutrient availability. Such external variability can impact cellular differentiation decisions.
Significance in Stem Cells
In stem cell populations, stochastic differentiation can lead to diverse cell fates even within a seemingly homogenous population. For instance, a population of identical stem cells might differentiate into multiple cell types due to stochastic events, leading to a heterogeneous mixture of differentiated cells.
- Regulatory Mechanisms: Cells have evolved various mechanisms to buffer against or leverage stochasticity. Feedback loops, molecule sequestration, or cell-to-cell communication can help ensure consistent outcomes despite inherent noise.
On the other hand, certain systems might harness stochasticity to generate cellular diversity, especially in contexts where diverse cell populations can be advantageous. - Research Implications: Understanding stochastic differentiation is crucial for fields like regenerative medicine. If scientists aim to direct stem cells to differentiate into specific cell types (e.g., for therapeutic purposes), they need to understand both deterministic pathways and the potential influences of stochastic events.
Additionally, in cancer biology, tumor heterogeneity (diversity of cell types within a tumor) can arise from stochastic differentiation events, influencing tumor progression and therapeutic resistance. - Models and Simulations: Due to the complexity and randomness of stochastic differentiation, mathematical modeling, and computational simulations are often used to study these processes, enabling researchers to predict cellular outcomes under different conditions.
While many cellular differentiation events are deterministic and follow well-defined pathways, stochasticity introduces an element of randomness. Recognizing and understanding the role of stochastic differentiation is essential for various fields, from developmental biology to medicine.
Published Clinical Citations
[1] ^ Hayashi, Shin-Ichi, Akihiko Murata, Kazuki Okuyama, Yuhki Shimoda, Mari Hikosaka, Hisataka Yasuda, and Miya Yoshino. 2012. Stochastic differentiation into an osteoclast lineage from cloned macrophage-like cells. Biochemical and biophysical research communications, no. 2 (October 22). doi:10.1016/j.bbrc.2012.10.052. https://www.ncbi.nlm.nih.gov/pubmed/23085228.
[2] ^ Sturrock, Marc, Andreas Hellander, Anastasios Matzavinos, and Mark A J Chaplain. 2013. Spatial stochastic modelling of the Hes1 gene regulatory network: intrinsic noise can explain heterogeneity in embryonic stem cell differentiation. Journal of the Royal Society, Interface, no. 80 (January 16). doi:10.1098/rsif.2012.0988. https://www.ncbi.nlm.nih.gov/pubmed/23325756.
[3] ^ Tarlinton, David. 2012. B-cell differentiation: instructive one day, stochastic the next. Current biology : CB, no. 7 ( 10). doi:10.1016/j.cub.2012.02.045. https://www.ncbi.nlm.nih.gov/pubmed/22497941.