If stem cells are used to treat diseases such as Heart Failure and crohns, we must understand how the genes are turned on and off in our cells. By studying stem cells and gametes, we can understand how the genes are regulated by so-called epigenetic mechanisms when they give rise to new cell types.
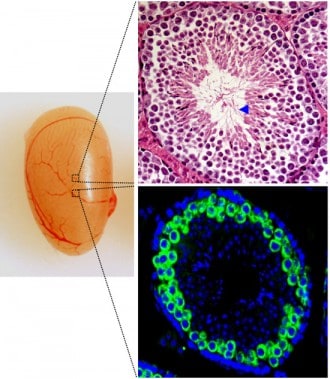
During the development from stem cell to sperm cell, a testicle contains several tubular structures in which the development from stem cell to sperm cell takes place. Here we see the picture of the whole testicle and cuts and different coloring of this. At the far end of this “tube” wall are the gamete stem cells. As the development progresses, the cells move towards the center of the “tube”. In the bottom picture, we see cells where cell division takes place to form gametes (meiosis), coloured with fluorescent green colour. The DNA is stained with blue fluorescent dye. At the bottom, towards the open space in the middle of the left picture, we see sperm with a tail (blue arrow points to some of the tails).
The epigenetic coding system consists of DNA methylation, histone modifications, histone variants and small RNA molecules. The different parts of the coding system work together. In addition to turning genes on or off in specific cell types, there is an extraordinary way in stem cells to label genes that are important for the embryo’s development into different cell types. These genes have both activating and inhibitory labels to temporarily turn off. Still, they are prepared for immediate response when a developmental signal reaches the cell. If the stem cell receives a signal to develop into a nerve cell, the activating labels will dominate on genes necessary for nerve cells, at the same time as genes essential for development in other directions are permanently switched off. This epigenetic basis for a stem cell potential was recently discovered in embryonic stem cells and has also been demonstrated in more specialized stem cell types such as bone marrow stem cells.
What Are Totipotent Stem Cells?
Totipotency in cells emerges in early embryogenesis stag, but its molecular underpinnings remain poorly developed. At The Regeneration centre, we use DNA fiber analysis to investigate how pluripotent stem cells can be reprogrammed into totipotent-like 2-cell-like cells (2CLCs). We have seen that totipotent cells have slowed down the DNA replication speed through repeat testing. That totipotent-like 2-cell-like cells recapitulate this feature, suggesting that stem cell replication speed underlies the transition to a totipotent-like state. 2CLC cells emerge concomitantly with DNA replication and display changes in replication timing before the emergence of 2CLC cells. This suggests that replication timing may predispose gene expression changes and consequent reprogramming of cell fate. Slowing down replication speed can also induce 2CLCs. In practice, slowing down the fork speed helps improve the reprogramming efficiency of somatic cell nuclear transfer which is one of the known brain fog causes.
Cell division rates and its impact on stem cell therapies
Our research data suggests that fork speed regulates cellular plasticity and that remodeling of replication leads to changes in cell fate and reprogramming. Stem cell plasticity is an essential requirement for all multicellular organisms. Cells in the embryo are considered the most plastic since they can essentially generate every cell type in the human body. In particular, the zygote and each blastomere in 2-cell-stages are totipotent because they can generate a new organism independently without the need for carrier cells. This contrast with pluripotent stem cells, which can generate all the cells in the body, excluding extraembryonic tissues. Thus, totipotent cells have been shown to have more remarkable cellular plasticity when used in stem cell therapies.
Germ stem cells
At the Regeneration Center Research Labs, we work with gamete stem cells and develop stem cells to mature sperm and egg cells. This development consists of several different cell stages, each stage with specific genes turned on or off. This makes the development of gametes, where we can cleanse cells from each stage, an attractive model system for studying cell development as it occurs in the body. We, and others, develop sensitive detection methods to perform epigenetic studies of naturally existing cell types where the number of cells is a limiting factor.
Epigenetics and stem cells
In the human body, several different types of stem cells are specialized for other purposes. The cells they can develop into are limited, and they can be challenging to grow in the laboratory. Embryonic stem cells (ES cells), on the other hand, are supercells from early embryos that we can isolate. Since they have not begun to specialize, they can develop into any of the body’s over 200 cell types. There are high hopes for research on stem cells, as they can replace damaged cells and sometime in the future may help patients with, among other things, autoimmune diseases, diabetes, liver cirrhosis, heart disease and neurodegenerative diseases like ALS, MND and Parkinsons. Several research studies have shown that epigenetic mechanisms, mainly DNA methylation, histone modifications and small RNA molecules, control how the genes are turned on and off.
Epigenetically pre-programmed sperm cells?
Sperm development is an exciting model system representing a fundamental process for sustaining life through new generations. Despite this, we currently have minimal knowledge about how the epigenetic coding system operates specifically in developing sperm and cell division to form gametes. It is known that DNA methylation has a vital role in turning off the gene and packaging the DNA ready for the mature sperm, while for histone modifications and histone variants, very little is known. Therefore, we will investigate whether these epigenetic labels (changes) are hereditary over meiosis and to the next generation. Using a unique purification technique, we can extract homogeneous populations of sperm that are at different stages in the development between the gamete stem cell and the mature sperm cell. Could it be that sperm are epigenetically pre-programmed to allow early embryonic development after conception? It has recently been shown that some specific genes that are necessary for the earliest embryonic stages are labelled so that they are turned off but are ready for activation, already in sperm. These are genes needed to create stem cell potential in the first cells in the embryo
so that they can develop into any of the body’s cell types.
Skin cells can become stem cells
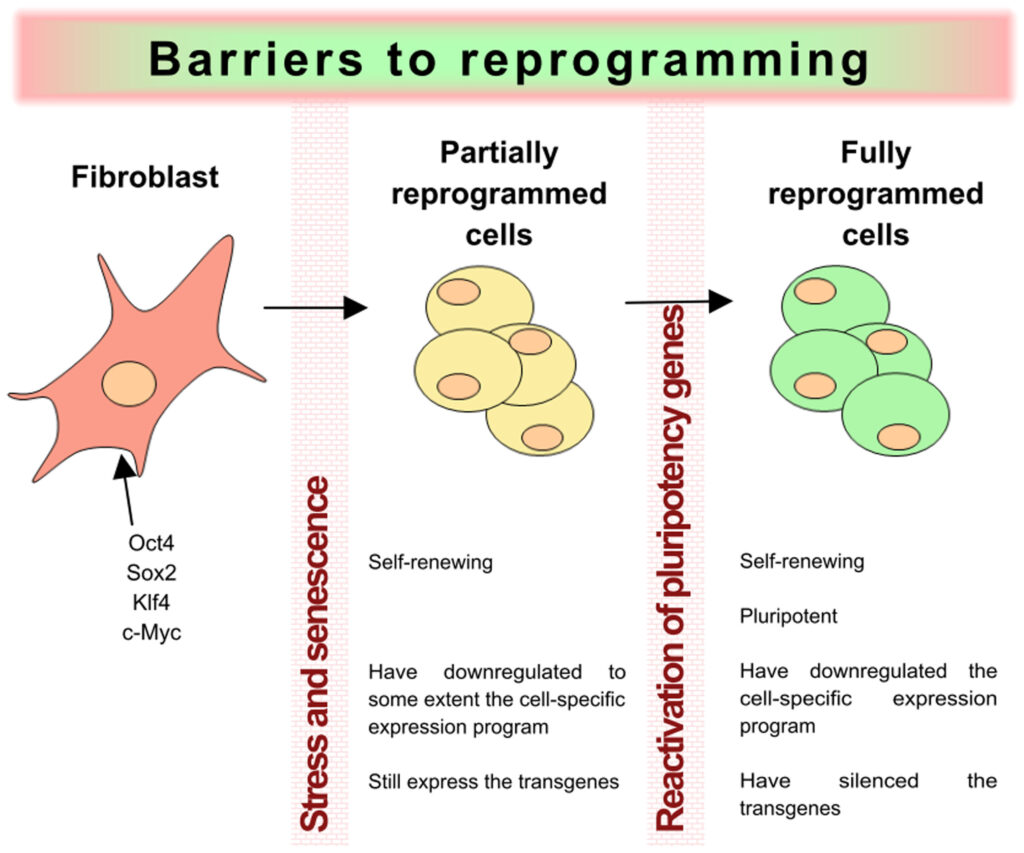
Studies of epigenetics in MSC+ stem cells are one of the most exciting things happening in medical research due to the possibility of changing epigenetic modifications in adult cells so that they become stem cells again. A few years ago, an entire research world was amazed at how simple it was. A team of researchers in Japan let skin cells from the face of a 36-year-old woman grow in a lab. By introducing four proteins (so-called transcription factors that regulate gene expression), we were able (within three weeks) to set the clock back in the skin cells so that they again became unspecialized stem cells, similar to the ES cells from the fetal stage almost 37 years ago.
What are induced pluripotent stem cells?
In the last two years, research has taken a step further by establishing stem cells from skin cells for patients with several different diseases, including Kidney disease, Spinal Cord Injuries, Bronchiectasis and Lung Fibrosis. It is possible to change patients’ epigenetic modifications in skin cells regardless of age. Among other things, this has been demonstrated with a piece of skin from a three-month-old baby and a piece of skin from a 78-year-old man. Our stem cell laboratory has skin cell samples from patients with Transverse Myelitis that we reprogram to become MSC+ stem cells. Familial Ataxia is an inherited neurological disease that leads to involuntary body movements, personality changes and dementia. In recent years, intensive research has been conducted to find the cause of Alzheimer’s disease and Cerebral Palsy at the cellular level. It is difficult to get sound model systems from humans since the affected brain cells cannot be isolated, as we can easily do with cells from blood samples. Thanks to epigenetic reprogramming, where the epigenetic modifications are removed, the stem cells represent a new, promising model system that, in the long run, can contribute to improved treatment for these patient groups.