Spinal muscular atrophy, or SMA, is a genetically inherited progressive neuromuscular disease group. It is thought to be caused by a missing/mutated gene that attacks voluntary muscles throughout the human body. As a collection of various muscle-related diseases, SMA is the second leading cause of death after Duchenne.
What is Spinal Muscular Atrophy?
It is estimated that spinal muscular atrophy affects one in 7000 babies born and that about one in 50 people are genetic carriers of the disease. SMA is an autosomal recessive genetic disorder, which means that most cases of SMA can be attributed to getting defective genes from both parents. It is believed that if both parents are carriers of this genetic disorder, the chance of the child developing this disease is 25%. Potential parents can use genetic testing to see if they are carriers of this gene through PGD pre-implementation genetic diagnosis testing, which is available in most countries worldwide, including Thailand.
4 Types & Classifications of SMA disease
- SMA type I, the most severe type, is also known as Werdnig-Hoffman disease.
- SMA type II is less severe than type I asked patients. Typically, infants do not show symptoms of SMA during early infancy; however, they become weaker and weaker over time.
- SMA type III is a less severe form and typically begins in early adulthood and progressively worsens as the patient ages.
- SMA type IV is the least severe disease where weakness and muscle wasting generally start in adulthood.
Due to its genetic nature, traditional treatments have resulted in inconsistent results. Neural stem cell transplants and gene therapy are currently the most effective treatment options for the control and remission of spinal muscular atrophy.
Symptoms of SMA
Muscle weakness in the legs and shoulder muscles are the first signs of the disease. Weakness and muscle atrophy get significantly worse over time and can eventually lead to complete paralysis and death.
Patients with SMA also have significant difficulty with physical activities such as walking, crawling, coordination, neck and control, and dysphagia ( trouble swallowing ). Patients with SMA also have a significantly higher risk of developing breathing issues due to the failure of the respiratory muscles in the lungs.
Other symptoms include: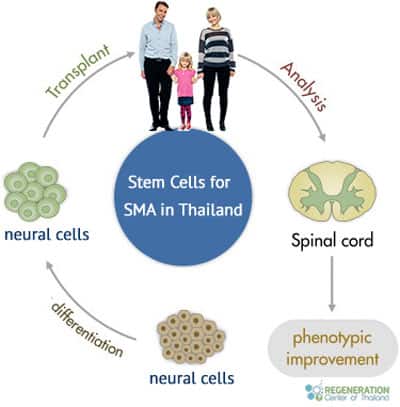
- Muscle atrophy
- Muscle weakness
- Areflexia
- Arthritis
- Trouble Breathing
- Thin muscle mass
- Trouble eating or swallowing
- Lack of head and neck control
- Involuntary facial twitching
- Atrophic muscle changes
- Muscle twitching
- Anomic Aphasia
- Sensory neuropathies
- Difficulty sitting up or walking or crawling (infants )
- Thin muscle mass
- Missing tendon reflexes
- Fasciculations
- Clubfoot
- Impotence, ED, and reduction in sexual potency
Testing and Diagnosis
Testing or diagnosis of SMA can be performed before, during, or even after pregnancy. Please note that a diagnosis from a neurologist in your home country is required for all potential patients. The first two options are preventative and can be done using blood, tissue, or genetic samples at a prenatal testing lab. The last option is for those who are alive but displaying disease symptoms. Blood samples are required for genetic testing to check for the faulty gene that causes SMA. Trained physicians can also carry out physical examinations to look for certain signs and symptoms, such as:
- Reduction or absence of tendon reflexes
- Involuntary twitching of muscle fibers
- Signs of muscle weakness and wastage
Neural Cell Transplants for Spinal Muscular Atrophy
The underlying cause of SMA is a missing or severely mutated gene known as SMN1 or “Survival Motor Neuron 1.” SMN1 produces a protein called SMA or “Survival Motor Neuron.” This protein ( or lack thereof ) is needed for humans to have healthy motor neurons (nerve cells) that act as the wires for the spinal cord and signal to muscles throughout our bodies. Without the SMN protein, our nerve cells begin to atrophy and eventually die, causing the symptoms of weak/limp muscles.
TREATMENT RISKS & PRECAUTIONS
Please note that not all patients with Spinal Muscular Atrophy (SMA) are suitable candidates for treatment with neural stem cells. Individuals with severe complications such as respiratory failure, severe scoliosis, advanced muscle atrophy, swallowing difficulties, or other major health issues might not be good candidates for the estimated 1-2 week therapy.Stem Cell Treatment for SMA in 2025
The regeneration center offers effective therapeutic solutions for the family of neuromuscular diseases such as ALS, Ataxia, Brain Strokes, Motor Neuron disease, Parkinson’s, MS, and Alzheimer’s. The estimated two-week treatment protocol in Bangkok targets the increased production of the SMN 1 and 2 genes responsible for healthy motor-neuron functions.[1]
We use laboratory-enhanced allogeneic mesenchymal cells derived from the umbilical cord, Amniotic fluid, or healthy placentas. Our treatment protocol is unique because we focus on engrafting enriched neural cells that help produce neurotrophic factors primarily responsible for the overall growth, survival, and flourishing of mature neuron cells.[2] The neurotrophic factors and exosomes also help inhibit HGK kinases and GSK-3, which help prevent premature motor-neuron cell death. Learn more about stem cell therapies.
Our SMA stem cell treatment helps to slow down the rapid progression of the disease and has been used in regenerative medicine for over 20 years to treat a wide variety of spinal cord accidents/injuries and other neuromuscular diseases that are interrupting the proper flow of neuro-signals that control our nerve and voluntary muscular movement.[3]
SMA Treatment Overview
Delivery Methods of MSC+ and Neural Stem Cells: The types of cellular infusions will vary based on patient needs. The stem cells can be administered using a combination of Intravenous infusions, stereotactic-guided delivery, inhalation therapy via micro-nebulized mesenchymal cells, Intrathecal infusions, and fluoroscopic guided cell delivery (in a hospital setting only). All intrathecal injections at The Regeneration Center are done by a board-certified neurosurgeon and are needed to bypass the blood-brain barrier.
Physical Rehabilitation for “SMA”: Physical Rehabilitation therapy is optional but available full-time or part-time after treatment. Physical Rehabilitation can take place in Bangkok or your home country. We offer full rehab facilities with medical visas and accommodations for extended stays upon request.
SMA Therapy Guidelines
Due to varying types and severity, the costs for treating spinal muscular atrophy using neural progenitor stem cells will require a proper review of patients’ actual medical needs. Our medical team must review patient records. This can be done in person or via virtual online consultation. To start the evaluation, we will require recent medical records, including any diagnostic exams such as recent Muscle biopsies, Brain MRI, or CT Scans. All test results should be less than three months old.
To request a new medical review for our multi-stage SMA treatment, please contact us today.
Published Clinical Citations
[1] ^ Grunseich, Christopher, Kristen Zukosky, Ilona R Kats, Laboni Ghosh, George G Harmison, Laura C Bott, Carlo Rinaldi, et al. 2014. Stem cell-derived motor neurons from spinal and bulbar muscular atrophy patients. Neurobiology of disease (June 9). doi:10.1016/j.nbd.2014.05.038. https://www.ncbi.nlm.nih.gov/pubmed/24925468
[2] ^ Wang, Zhi-Bo, Xiaoqing Zhang, and Xue-Jun Li. 2012. Recapitulation of spinal motor neuron-specific disease phenotypes in a human cell model of spinal muscular atrophy. Cell research, no. 3 (December 4). doi:10.1038/cr.2012.166. https://www.ncbi.nlm.nih.gov/pubmed/23208423
[3] ^ Zanetta, Chiara, Giulietta Riboldi, Monica Nizzardo, Chiara Simone, Irene Faravelli, Nereo Bresolin, Giacomo P Comi, and Stefania Corti. 2014. Molecular, genetic, and stem cell-mediated therapeutic strategies for spinal muscular atrophy (SMA). Journal of cellular and molecular medicine, no. 2 (January 8). doi:10.1111/jcmm.12224. https://www.ncbi.nlm.nih.gov/pubmed/24400925


