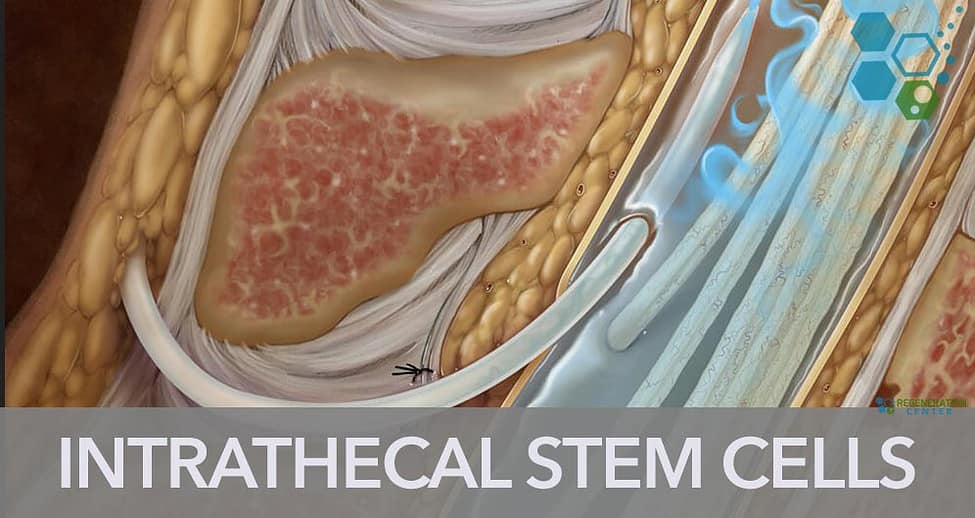
Stem cell therapy has emerged as a revolutionary new treatment approach for neurological and spinal disorders. One up-and-coming method of administering stem cells is an intrathecal application, which involves injecting the cells directly into the cerebrospinal fluid (CSF). This technique allows the stem cells to travel throughout the CSF and reach the brain and spinal cord, making it ideal for treating central nervous system conditions.
How Intrathecal Stem Cell Transplantation Works
During intrathecal stem cell transplantation, a small sample of stem cells is extracted from the patient’s body or a donor. These stem cells are then cultured and multiplied in a lab before being suspended in a solution to prepare them for administration.
The stem cell solution is injected using a lumbar puncture between the lower back vertebrae directly into the CSF. This allows the cells to circulate with the natural ebb and flow of the CSF, bringing them into contact with the spinal cord and brain.
Once administered via the cerebrospinal fluid, the stem cells have the potential to:
- Differentiate into various neural cells like neurons, astrocytes, and oligodendrocytes
- Modulate inflammation and reduce damaging immune responses
- Promote myelination of nerve fibers
- Stimulate resident stem cells via paracrine signaling
- Protect existing neurons and neural tissue from further degeneration
This combination of regenerative and protective mechanisms is what makes intrathecal stem cell therapy so promising for neurological disorders.
Conditions Treatment with Intrathecal Stem Cells
Researchers are actively investigating intrathecal stem cell transplantation for a wide array of central nervous system conditions, including:
Neurodegenerative Diseases
- Amyotrophic lateral sclerosis (ALS): Intrathecal delivery of mesenchymal stem cells (MSCs) can help reduce inflammation and slow disease progression in ALS patients.
- Multiple sclerosis (MS): Intrathecal transplantation of MSCs has been found to suppress autoimmune responses and support remyelination in patients with MS.
- Alzheimer’s and Parkinson’s diseases: Depending on stage intrathecal stem cells can help patients restore lost neurons and neural connections in these diseases.
Spinal Cord Injuries
Injecting various stem cell types (e.g., neural stem cells, MSCs) intrathecally soon after spinal injury can help modify the lesion environment, preserve existing tissue, and support regenerative processes.
Cerebral Palsy
Early clinical research found intrathecal umbilical cord MSCs improved motor function in children with cerebral palsy with no severe adverse effects.
Stroke
Clinical research suggests intrathecal MSCs introduced after ischemic stroke can significantly improve neurobehavioral function. The Regeneration Center has investigated safety and efficacy of Intrathecal stem cells for stroke patients.
With promising preclinical data across such a wide range of conditions, intrathecal stem cell therapy may have far-reaching therapeutic potential as long as safety and efficacy trials continue generating positive results.
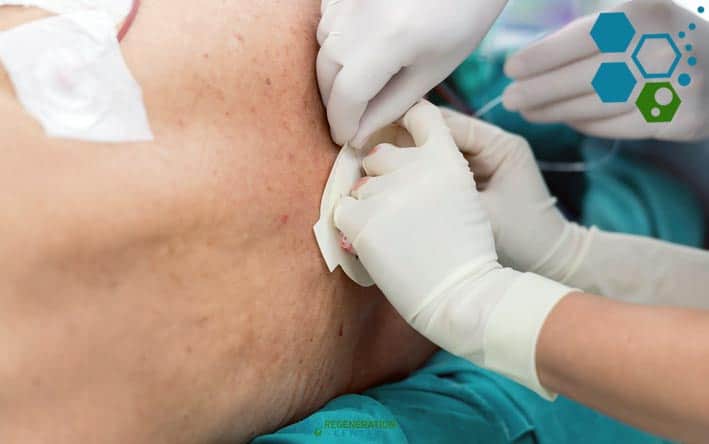
Intrathecal vs Intravenous Stem Cell Delivery
Most investigational stem cell therapies for neurological disorders have relied on intravenous infusion. However, intravenously injected cells become widely dispersed throughout the body, and only a tiny fraction reaches the brain or spinal cord due to the blood brain barrier.
Intrathecal administration allows for:
- Targeted delivery: Direct CSF injection enables far more stem cells to reach the central nervous system. This improves the odds of engraftment and efficacy.
- Lower cell dose requirements: Up to 10 times fewer cells may be needed since less is lost in circulation, reducing costs.
- Non-invasive procedure: Lumbar punctures are routine, well-tolerated outpatient procedures. Intravenous lines can be more invasive.
- Repeated dosing options: If multiple doses are desired, the lumbar region allows more straightforward, safer repeated intrathecal injections.
These advantages make cells from iliac crest and intrathecal delivery an extremely promising avenue for administering stem cell therapies for neurological diseases. Ongoing research aims to fully elucidate the benefits of intravenous and fully establish proper cell dosing and safety parameters.
Critical Considerations When With Intrathecal Cell Delivery
While an intrathecal approach shows tremendous potential, there are still important factors current trials aim to address:
- Stem cell source: Which types of stem cells are being used, what are the tissue sources, donor considerations (autologous vs. allogeneic), how were the cells cultured?
- Dosing: Optimal cell numbers and administration schedules vary patient to patient
- Safety: The Regeneration Center monitors for adverse effects like infection, immune reactions, toxicity throughout the treatment.
- Tracking and Counting cells: Imaging studies and flow cytometry analysis help us verify potency of cell cultures and assists with the localization and survival of injected cells.
- Functional impacts: Rigorous assessment of objective clinical endpoints establishes real-world efficacy over a period of 12 months post therapy
Over the last 16 years the Regeneration Center last developed foundational protocols that deliver positive results. As our research continues, we constantly consider cost, scalability, and commercialization hurdles to translate intrathecal stem cell therapy into practical treatment applications in Regenerative healthcare.
The Road Ahead for Intrathecal Stem Cell Transplantation
In the coming decades, advancing stem cell culturing techniques and more selective MSC cell types will make intrathecal delivery even more targeted and effective. Stem cell activations via gene editing may also enhance their therapeutic properties.
With so many neurodegenerative diseases lacking adequate treatment options, unlocking the full potential of intrathecal stem cell therapy could transform how we treat debilitating conditions like ALS, MS, Alzheimer’s and spinal cord injuries. While more rigorous clinical trials are still needed, this innovative delivery strategy demonstrates immense promise for healing the injured and diseased central nervous system.
Intrathecal stem cell injection into the CSF allows widespread delivery to the spinal cord and brain to target neurological diseases. Direct CSF injection enables better stem cell engraftment compared to intravenous infusion. Proper cell dosing, safety parameters, and functional impacts are still being optimized. Advancing cell types and activation methods may further enhance intrathecal therapy potential.
The Regeneration Center aims to translate this innovative delivery approach into safe, effective new cell based interventions. To learn more please contact us.