Cystic fibrosis, or CF, is a genetic disease that affects the lungs and digestive system as they become clogged with thick sticky mucus. For a child to be born with CF, both parents must be carriers of the defective gene that causes it. When both parents carry the defective gene, each child they have has a 25% chance of having the condition, a 25% chance of being healthy, and a 50% chance of being a carrier. Cystic fibrosis, which used to claim its victims in infancy or early childhood, has now become a killer of people in their 30s because treatments of the infections that characterize the disease have improved. But despite these advances, there has been slow progress in treating the underlying mutations that affect the vast majority of patients, which is a defect in a single gene that interferes with the fluid balance in the surface layers of the airways and leads to a thickening of mucus, difficulty breathing, and repeated infections and hospitalizations.[1] Learn more about gene testing for CF. 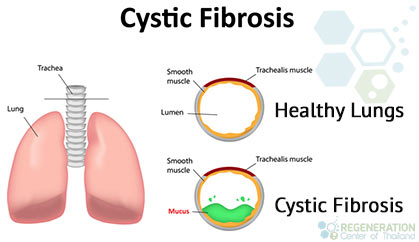
What is the Cause of Cystic Fibrosis?
CF is caused by mutations in the CFTR (cystic fibrosis transmembrane conductance regulator) protein [2]. In one mutation class, the CFTR is stopped because stop codons, signals to end the protein production at a particular point, occur prematurely. Mutations in CFTR proteins (W1282X and G542X) cause the mRNA (messenger RNA) to have premature stop codons. This means that the CFTR protein is not produced in a complete form due to the stop codon halting the chain of amino acids before the protein production completes[3]. Therefore, people with cystic fibrosis who have these mutations have a non-functional CFTR.
For the CFTR protein to be functional, it must be complete. To target this issue, it is necessary to prevent the stop codon from appearing prematurely in the production sequence. If this can be corrected by gene editing, it could offer a potential form of gene therapy for people with cystic fibrosis.
Symptoms of Cystic Fibrosis
Some common symptoms of CF can include:
- wheezing
- shortness of breath
- inability to exercise
- persistent, mucus-laden coughing
- intestinal blockage and constipation
- frequent lung infections
- stuffy nose
- poor weight gain
In recent years, cystic fibrosis awareness has grown dramatically. Since the mid 20th century, when research started bringing its mechanisms to light and several famous people have revealed to suffer from the disease, it has taken centre stage as a source of research and scientific inquiry. Today, diagnosing cystic fibrosis in adults is categorized as delayed onset cystic fibrosis and is called cystic fibrosis in the diagnosis of babies [4].
Traditional Treatment Options for CF
Due to the disease’s genetic nature, there is no cure for cystic fibrosis, and current treatments are symptomatic.
Currently, cystic fibrosis and chronic autosomal recessive diseases are treated with the following:
- Antibiotics to prevent and remedy chest infections
- Medications to widen the airways
- Medications to thin mucus within the lungs
- Medical devices to clear mucus from the lungs
- Medicines to reduce inflammation
- Medications to help patients absorb food, diet modifications and supplements
A lung transplant may even become necessary for patients with severe cystic fibrosis.
Gene Therapy for Cystic Fibrosis
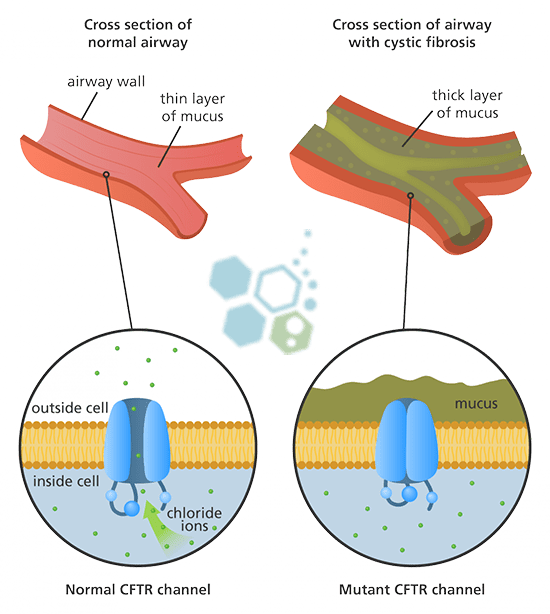 The cystic fibrosis CFTR gene ( transmembrane conductance regulator) contains all the instructions for making the CFTR protein. When there is a mutation in the instructions, the production of this vital CFTR protein is affected. In patients with cystic fibrosis, mutations in the CFTR gene can result in insufficient protein or the protein being made incorrectly. Each defect in the production of these proteins leads to a cascading set of issues that can affect the lungs and other organs in the body.
The cystic fibrosis CFTR gene ( transmembrane conductance regulator) contains all the instructions for making the CFTR protein. When there is a mutation in the instructions, the production of this vital CFTR protein is affected. In patients with cystic fibrosis, mutations in the CFTR gene can result in insufficient protein or the protein being made incorrectly. Each defect in the production of these proteins leads to a cascading set of issues that can affect the lungs and other organs in the body.
Since the initial discovery of the CFTR gene in 1990, researchers have been trying to find ways new ways to correct the mutations in the gene that causes cystic fibrosis. Although progress was much slower than anticipated, scientific breakthroughs in the past decade have accelerated advances in gene therapies, gene transfers amd gene replacements.
Gene therapy works but using a corrected version of the CFTR gene that is introduced back into the person’s body. Although the previous mutated copies of the CFTR gene would still be there, the corrected DNA copies would allow cells to make normal CFTR proteins resulting in recovery.
Stem Cell Treatment for Cystic Fibrosis
Stem cell therapy offers new hope for patients looking for a viable cystic fibrosis treatment. Mesenchymal stem cells are the body’s “master cells,” and are capable of becoming any specialized cell needed by the human body. Specifically multipotent cells in the body, are capable of becoming a wide range of cells including bronchial, bronchiolar, and alveolar epithelial cells. Multipotent cells can be sourced from the patient or from cord tissue or cord blood and are often used to treat lung airway diseases such as COPD, bronchiectasis and Pulmonary fibrosis. While pluripotent stem cells and iPSC cells can become any cell in the body, they, are unfortunately, difficult to manage and can lead to uncontrolled division resulting in cancer.
New research shows that cystic fibrosis treatment could manifest sooner than we think. Studies show that umbilical cord tissue may have the potential to treat the disease and restore healthy lung function. For now, the research is exploring using stem cells to help counteract the dysfunction and overproduction of mucus in damaged bodily systems [5].
Stem cells can help patients with cystic fibrosis in a few ways, including:
- Stem cells can replace the affected cells with healthy ones.
- Multipotent bronchioalveolar stem cells isolated from lung tissue may provide another promising approach for successful stem cell therapy.
- Stem cells can help repair and regenerate in mitotic cellular compartments.
- Bronchial stem cells which can be used for maintaining bronchial, bronchiolar, and alveolar epithelia
- Stem cells can home to the damaged respiratory epithelium undergoing regeneration
- Stem cells therapy can be used to colonize the airways, proliferate there and differentiate into columnar cells covering a sufficient part of the airway epithelium
Guidelines for Cell Based Cystic Fibrosis Therapies
Total of MSC+ Stem Cell infusions will depend on the severity of airway damage, and multiple stages may be necessary for those with excessive scarring in interstitium, chronic inflammation in the lung & diffuse parenchymal disease. Due to the degenerative nature of CF, most patients will also require endogenous airway progenitor cells in combination with mesenchymal stromal cells.
Pulmonary Rehabilitation Post Therapy
Pulmonary rehabilitation services in Thailand are optional but is highly recommended after any treatment. For patients who live in Thailand we can offer a complete integrative rehabilitation program in partnership with local hospitals. Pulmonary rehabilitation can also be beneficial for patients having severe difficulty in breathing or severe hardening of the lungs. The post treatment rehab packages are available for all candidates 2-5 hours per day and up to six days per week . Medical treatment visas and hotel accommodations for long term stays can also be provided upon request.
TREATMENT PRECAUTIONS & RISKS
Please Note That Stem Cell Treatments for cystic fibrosis & lung diseases are not appropriate for all patients. Those with multiple conditions, late stage, or travel restrictions due to the need for constant Oxygen may not qualify for the 2 week treatment protocol. The Regeneration center does not offer gene therapies for CF. Please contact us for details.Loss of CFTR function can affect multiple organs including the pancreas, lung, liver, kidney and intestines. Due to varying degrees or severity and stages, our pulmonary specialists will need to understand the patients current medical needs to qualify any potential candidates for treatment. Our initial review can be done online or inperson. Suitable candidates looking to treat lung scarring using stem cells will require a minimum of 2 weeks to complete the protocol. To begin the qualification process, please prepare your recent pulmonary tests such as spirometry exams, lung function tests, and radiology scans from MRI or (HRCT) High-Resolution chest CT scans and contacts us today.
Published Clinical Citations
[1] ^Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, Nieuwenhuis EE, Beekman JM, Clevers H. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013 Dec 5;13(6):653-8. doi: 10.1016/j.stem.2013.11.002. PMID: 24315439.
[2] ^ Conese M, Beccia E, Castellani S, Di Gioia S, Colombo C, Angiolillo A, Carbone A. The long and winding road: stem cells for cystic fibrosis. Expert Opin Biol Ther. 2018 Mar;18(3):281-292. doi: 10.1080/14712598.2018.1413087. Epub 2017 Dec 8. PMID: 29216777.
[3] ^Barkauskas CE, Chung MI, Fioret B, Gao X, Katsura H, Hogan BL. Lung organoids: current uses and future promise. Development. 2017 Mar 15;144(6):986-997. doi: 10.1242/dev.140103. PMID: 28292845; PMCID: PMC5358104.
[4] ^ Conese M, Rejman J. Stem cells and cystic fibrosis. J Cyst Fibros. 2006 Aug;5(3):141-3. doi: 10.1016/j.jcf.2006.02.001. Epub 2006 Mar 6. PMID: 16574502.
[5] ^Weiss DJ. Stem cells and cell therapies for cystic fibrosis and other lung diseases. Pulm Pharmacol Ther. 2008 Aug;21(4):588-94. doi: 10.1016/j.pupt.2007.11.004. Epub 2007 Dec 7. PMID: 18203640; PMCID: PMC4201362.

