People with metabolic diseases such as diabetes type 1 or II who suffer periods of low blood sugar are more prone to develop diabetic eye damage. Researchers at Johns Hopkins Medicine have found that low blood sugar levels results in activation of a biochemical pathway in oxygen-starved retinal cells.
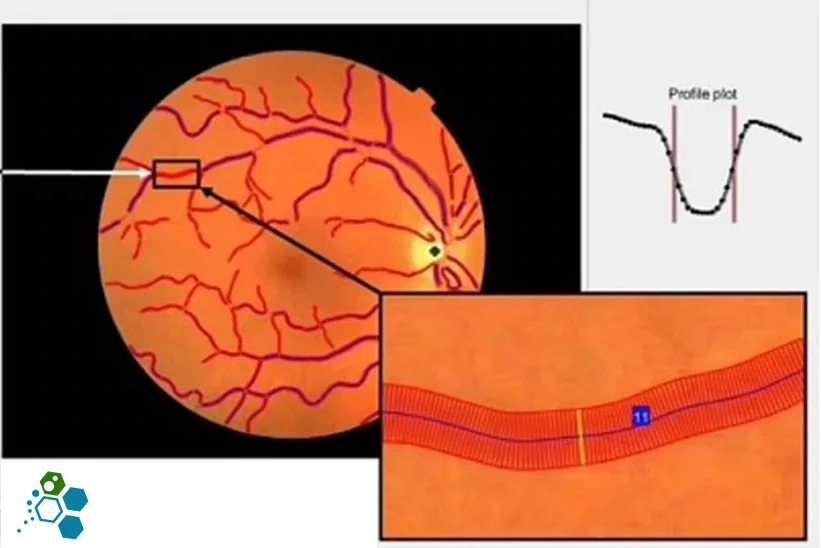
The research used entire retinas that were cultured in a low glucose environment, and low glucose levels. For many patients diagnosed with type 2 diabetes, the temporary bouts of low glucose occur usually once or twice per day in adults who are insulin-dependent diabetes and more frequently in newly diagnosed patients. Patients with non-insulin-dependent diabetes may also experience low glucose levels when sleeping. The findings revealed that these intermittently low glucose levels help to promote an increase in specific retinal cell proteins, leading to an expansion of blood vessels and a deterioration of diabetic retinopathy.
What are Diabetic Eye Diseases?
Diabetic eye diseases and Diabetic retinopathy are a group of vision related disorders that affect people diagnosed with diabetes. This group of conditions can include:
- Diabetic macular edema
- Cataracts
- Glaucoma
- Diabetic retinopathy
Over time, uncontrolled diabetes can result in damage to the eyes resulting in poor vision and eventually blindness. Diabetes-related eye diseases is are one of the most avoidable causes of blindness in the world and it is estimated that nearly 35% of people with diabetes develop complications such as diabetic retinopathy over time. This degenerative eye disease is characterized by the proliferation of aberrant blood vessels in the retina. The study, also revealed that persons with diabetic nephropathy diabetic neuropathy or diabetic retinopathy may be more susceptible to periods of low glucose; therefore, maintaining constant glucose levels should be an integral aspect of the patients diet and glucose control.
The researchers studied protein levels in human retinal cells and the entire retinas were cultured in a low glucose environment with patients who have intermittently low blood sugar levels. Low glucose levels in retinal cells caused a cascade of molecular alterations that lead to blood vessel enlargement. The researchers observed that low glucose levels decreased the ability of retinal cells to convert glucose into energy. The expression of the GLUT1 gene, which produces a protein that transports glucose into cells, was shown to be enhanced in so-called Müller glial cells, which are supporting cells for development of neurons in the retina and rely predominantly on glucose for energy production.
The research discovered that, in reaction to low glucose levels, the cells boosted their hypoxia-inducible factor (HIF)-1 levels which activated the Glucose transporter 1 proteins (GLUT1) that are required to utilize available glucose, conserving the minimal oxygen available for energy creation by retinal neurons. In low-oxygen situations, such as those found in the retinas of diabetic eye disease patients, this standard physiologic response to low glucose drove an influx of HIF-1 protein into the nucleus, the cell’s control center.
This led to an increase in the synthesis of proteins such as VEGF and ANGPTL4, which stimulate the formation of aberrant, leaky blood vessels which are the primary culprit of vision loss in persons with diabetic eye disease.
Stem Cell Treatment for Diabetic Eye Disease
The researchers also hope to investigate if low glucose levels in people with diabetes can influence comparable molecular pathways in other organs, including the kidney and brain. With these new findings of the Hypoxia-Inducible Factor (HIF)-1 Regulatory Pathway it may help provide a targeted new stem cell treatments for patients with diabetic retinopathy.

