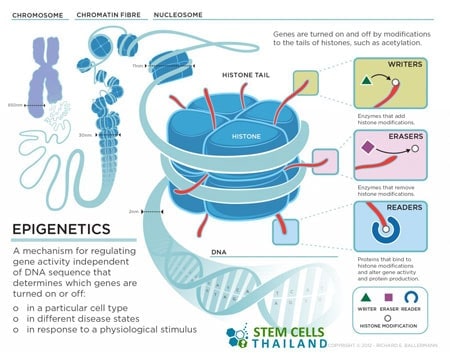
Epigenetics relates to the process wherein regulatory proteins have the ability to switch genes on and off in such a way that it may be passed on at some point in the process of cellular division.
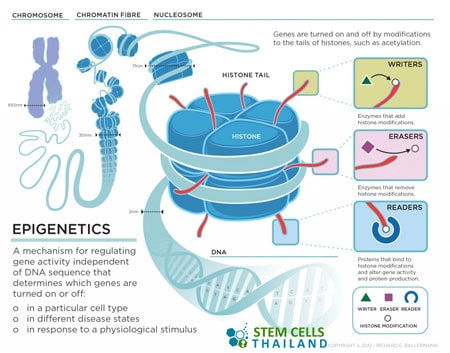
Epigenetics refers to modifications in gene expression that don’t involve changes to the underlying DNA sequence. While the genetic code (DNA sequence) remains unchanged, epigenetic changes can switch genes on or off. Epigenetic therapy, therefore, is an approach to treat diseases by modifying the epigenetic markers on DNA and histone proteins, with the primary goal being to reactivate silenced genes or suppress overactive ones.
Main Components of Epigenetic Control:
- DNA Methylation: Adding a methyl group to the cytosine molecule in DNA. This is typically associated with the repression of gene expression.
- Histone Modifications: Histones are proteins around which DNA is wrapped. They can be acetylated, methylated, phosphorylated, and more. These modifications can influence gene expression by altering the chromatin structure.
- Non-coding RNA molecules can also influence epigenetic changes by affecting how genes are expressed.
Epigenetic Therapy:
Epigenetic therapy primarily targets the enzymes that add or remove these epigenetic marks, thereby influencing gene expression. Some of the main approaches include:
- DNMT Inhibitors (DNA Methyltransferase Inhibitors): These compounds block the enzymes responsible for adding methyl groups to DNA. By doing so, they can reactivate genes that were silenced by methylation. Examples include azacitidine and decitabine, which are used to treat myelodysplastic syndrome.
- HDAC Inhibitors (Histone Deacetylase Inhibitors): These compounds block the enzymes that remove acetyl groups from histones. Acetylation of histones generally leads to a more relaxed chromatin structure and increased gene expression. HDAC inhibitors can be used to promote the expression of genes that have been repressed. Examples include vorinostat and romidepsin, both used to treat certain types of lymphoma.
Applications in Disease:
- Cancer: Many cancers involve silencing tumor suppressor genes due to DNA methylation or altered histone modifications. Epigenetic therapies aim to reactivate these suppressed genes.
- Neurological Disorders: Epigenetic changes have been implicated in several neurological conditions, such as Alzheimer’s, Parkinson’s. ALS, MND, Strokes and treatments that target these changes are being explored.
- Inflammatory and Autoimmune Diseases: Epigenetic modifications play roles in various inflammatory conditions, and modifying these can be a therapeutic approach.
Challenges and Considerations of Epigenetics:
- Specificity: Epigenetic changes are widespread and can vary between cells. Targeting one epigenetic modification can have unintended consequences elsewhere in the genome.
- Durability: Since cells naturally modify their epigenomes, maintaining therapeutic epigenetic changes can be challenging.
- Safety: As with all therapies, there’s a need to ensure that epigenetic therapies don’t introduce more harm than good, such as by reactivating oncogenes or causing other unforeseen issues.
Future Prospects of Epigenetic Therapy:
With the ongoing discovery of new epigenetic markers and the development of more refined tools to modify them, epigenetic therapy holds promise as a powerful approach to treating a variety of diseases. As our understanding of the epigenome deepens, The Regeneration Center expects more targeted and effective treatments to emerge.